Abstract
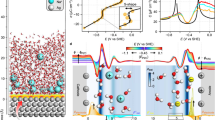
The capacitance of the electrochemical interface has traditionally been separated into two distinct types: non-Faradaic electric double-layer capacitance, which involves charge induction, and Faradaic pseudocapacitance, which involves charge transfer. However, the electrochemical interface in most energy technologies is not planar but involves porous and layered materials that offer varying degrees of electrolyte confinement. We suggest that understanding electrosorption under confinement in porous and layered materials requires a more nuanced view of the capacitive mechanism than that at a planar interface. In particular, we consider the crucial role of the electrolyte confinement in these systems to reconcile different viewpoints on electrochemical capacitance. We propose that there is a continuum between double-layer capacitance and Faradaic intercalation that is dependent on the specific confinement microenvironment. We also discuss open questions regarding electrochemical capacitance in porous and layered materials and how these lead to opportunities for future energy technologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ratajczak, P., Suss, M. E., Kaasik, F. & Béguin, F. Carbon electrodes for capacitive technologies. Energy Storage Mater. 16, 126–145 (2019).
Srimuk, P., Su, X., Yoon, J., Aurbach, D. & Presser, V. Charge-transfer materials for electrochemical water desalination, ion separation and the recovery of elements. Nat. Rev. Mater. 5, 517–538 (2020).
Conway, B. E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications (Kluwer-Academic, 1999).
Simon, P., Gogotsi, Y. & Dunn, B. Where do batteries end and supercapacitors begin? Science 343, 1210–1211 (2014).
Brousse, T., Bélanger, D. & Long, J. W. To be or not to be pseudocapacitive? J. Electrochem. Soc. 162, A5185–A5189 (2015).
Costentin, C., Porter, T. R. & Savéant, J. M. How do pseudocapacitors store energy? Theoretical analysis and experimental illustration. ACS Appl. Mater. Interfaces 9, 8649–8658 (2017).
Costentin, C. & Savéant, J.-M. Energy storage: pseudocapacitance in prospect. Chem. Sci. 10, 5656–5666 (2019).
Mathis, T. S. et al. Energy storage data reporting in perspective—guidelines for interpreting the performance of electrochemical energy storage systems. Adv. Energy Mater. 9, 1902007 (2019).
Fleischmann, S. et al. Pseudocapacitance: from fundamental understanding to high power energy storage materials. Chem. Rev. 120, 6738–6782 (2020).
Gileadi, E. Electrode Kinetics for Chemists, Chemical Engineers, and Materials Scientists (VCH Publishers Inc., 1993).
Waegele, M. M., Gunathunge, C. M., Li, J. & Li, X. How cations affect the electric double layer and the rates and selectivity of electrocatalytic processes. J. Chem. Phys. 151, 160902 (2019).
Wang, X., Liu, K. & Wu, J. Demystifying the Stern layer at a metal–electrolyte interface: local dielectric constant, specific ion adsorption, and partial charge transfer. J. Chem. Phys. 154, 124701 (2021).
Eliad, L., Salitra, G., Soffer, A. & Aurbach, D. Ion sieving effects in the electrical double layer of porous carbon electrodes: estimating effective ion size in electrolytic solutions. J. Phys. Chem. B 105, 6880–6887 (2001).
Segalini, J., Daffos, B., Taberna, P. L., Gogotsi, Y. & Simon, P. Qualitative electrochemical impedance spectroscopy study of ion transport into sub-nanometer carbon pores in electrochemical double layer capacitor electrodes. Electrochim. Acta 55, 7489–7494 (2010).
Chmiola, J. et al. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 313, 1760–1763 (2006).
Dou, Q., Liu, L., Yang, B., Lang, J. & Yan, X. Silica-grafted ionic liquids for revealing the respective charging behaviors of cations and anions in supercapacitors. Nat. Commun. 8, 2188 (2017).
Biesheuvel, P. M., Porada, S., Levi, M. & Bazant, M. Z. Attractive forces in microporous carbon electrodes for capacitive deionization. J. Solid State Electrochem. 18, 1365–1376 (2014).
Lin, R. et al. Solvent effect on the ion adsorption from ionic liquid electrolyte into sub-nanometer carbon pores. Electrochim. Acta 54, 7025–7032 (2009).
Prehal, C. et al. Quantification of ion confinement and desolvation in nanoporous carbon supercapacitors with modelling and in situ X-ray scattering. Nat. Energy 2, 16215 (2017).
Zhan, C. et al. Computational insights into materials and interfaces for capacitive energy storage. Adv. Sci. 4, 1700059 (2017).
Jiang, J., Li, Y., Liu, J. & Huang, X. Building one-dimensional oxide nanostructure arrays on conductive metal substrates for lithium-ion battery anodes. Nanoscale 3, 45–58 (2011).
Jiang, D. & Wu, J. Microscopic insights into the electrochemical behavior of nonaqueous electrolytes in electric double-layer capacitors. J. Phys. Chem. Lett. 4, 1260–1267 (2013).
Kondrat, S., Perez, C. R., Presser, V., Gogotsi, Y. & Kornyshev, A. A. Effect of pore size and its dispersity on the energy storage in nanoporous supercapacitors. Energy Environ. Sci. 5, 6474–6479 (2012).
Largeot, C. et al. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 130, 2730–2731 (2008).
Kondrat, S., Georgi, N., Fedorov, M. V. & Kornyshev, A. A. A superionic state in nano-porous double-layer capacitors: insights from Monte Carlo simulations. Phys. Chem. Chem. Phys. 13, 11359–11366 (2011).
Futamura, R. et al. Partial breaking of the coulombic ordering of ionic liquids confined in carbon nanopores. Nat. Mater. 16, 1225–1232 (2017).
Redondo, E. et al. Outstanding room-temperature capacitance of biomass-derived microporous carbons in ionic liquid electrolyte. Electrochem. Commun. 79, 5–8 (2017).
Weingarth, D. et al. Graphitization as a universal tool to tailor the potential-dependent capacitance of carbon supercapacitors. Adv. Energy Mater. 4, 1400316 (2014).
Kornyshev, A. A., Luque, N. B. & Schmickler, W. Differential capacitance of ionic liquid interface with graphite: the story of two double layers. J. Solid State Electrochem. 18, 1345–1349 (2014).
Lee, H. Y. & Goodenough, J. B. Supercapacitor behavior with KCl electrolyte. J. Solid State Chem. 144, 220–223 (1999).
Nam, K. W., Kim, M. G. & Kim, K. B. In situ Mn K-edge X-ray absorption spectroscopy studies of electrodeposited manganese oxide films for electrochemical capacitors. J. Phys. Chem. C 111, 749–758 (2007).
Chen, D. et al. Probing the charge storage mechanism of a pseudocapacitive MnO2 electrode using in operando Raman spectroscopy. Chem. Mater. 27, 6608–6619 (2015).
Boyd, S. et al. Effects of interlayer confinement and hydration on capacitive charge storage in birnessite. Nat. Mater. 20, 1689–1694 (2021).
Naguib, M. et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 23, 4248–4253 (2011).
Quilty, C. D. et al. Ex situ and operando XRD and XAS analysis of MoS2: a lithiation study of bulk and nanosheet materials. ACS Appl. Energy Mater. 2, 7635–7646 (2019).
Bärmann, P. et al. Solvent co-intercalation into few-layered Ti3C2Tx MXenes in lithium ion batteries induced by acidic or basic post-treatment. ACS Nano 15, 3295–3308 (2021).
Wang, X. et al. Influences from solvents on charge storage in titanium carbide MXenes. Nat. Energy 4, 241–248 (2019).
Ando, Y., Okubo, M., Yamada, A. & Otani, M. Capacitive versus pseudocapacitive storage in MXene. Adv. Funct. Mater. 30, 2000820 (2020).
Hui, J., Burgess, M., Zhang, J. & Rodríguez-López, J. Layer number dependence of Li+ intercalation on few-layer graphene and electrochemical imaging of its solid–electrolyte interphase evolution. ACS Nano 10, 4248–4257 (2016).
Masel, R. I. Principles of Adsorption and Reaction on Solid Surfaces Vol. 3 (John Wiley & Sons, 1996).
Mitchell, J. B. et al. Confined interlayer water promotes structural stability for high-rate electrochemical proton intercalation in tungsten oxide hydrates. ACS Energy Lett. 4, 2805–2812 (2019).
Goubard-Bretesché, N. et al. Unveiling pseudocapacitive charge storage behavior in FeWO4 electrode material by operando X-ray absorption spectroscopy. Small 16, 2002855 (2020).
Forse, A. C. et al. Direct observation of ion dynamics in supercapacitor electrodes using in situ diffusion NMR spectroscopy. Nat. Energy 2, 16216 (2017).
Ma, H. et al. Maximization of spatial charge density: an approach to ultrahigh energy density of capacitive charge storage. Angew. Chem. 132, 14649–14657 (2020).
Robert, K. et al. Novel insights into the charge storage mechanism in pseudocapacitive vanadium nitride thick films. Energy Environ. Sci. 13, 949–957 (2020).
Bi, S. et al. Permselective ion electrosorption of subnanometer pores at high molar strength enables capacitive deionization of saline water. Sustain. Energy Fuels 4, 1285–1295 (2020).
Zhang, Y., Peng, J., Feng, G. & Presser, V. Hydration shell energy barrier differences of sub-nanometer carbon pores enable ion sieving and selective ion removal. Chem. Eng. J. 419, 129438 (2021).
Zhao, R. et al. Time-dependent ion selectivity in capacitive charging of porous electrodes. J. Colloid Interface Sci. 384, 38–44 (2012).
Sima, U. et al. MXene artificial muscles based on ionically cross-linked Ti3C2Tx electrode for kinetic soft robotics. Sci. Robot. 4, eaaw7797 (2019).
Tsai, W.-Y., Wang, R., Boyd, S., Augustyn, V. & Balke, N. Probing local electrochemistry via mechanical cyclic voltammetry curves. Nano Energy 81, 105592 (2021).
Jäckel, N., Patrick Emge, S., Krüner, B., Roling, B. & Presser, V. Quantitative information about electrosorption of ionic liquids in carbon nanopores from electrochemical dilatometry and quartz crystal microbalance measurements. J. Phys. Chem. C 121, 19120–19128 (2017).
Srivastava, D., Santiso, E. E. & Gubbins, K. E. Pressure enhancement in confined fluids: effect of molecular shape and fluid–wall interactions. Langmuir 33, 11231–11245 (2017).
Santiso, E. E., Kostov, M. K., George, A. M., Nardelli, M. B. & Gubbins, K. E. Confinement effects on chemical reactions—toward an integrated rational catalyst design. Appl. Surf. Sci. 253, 5570–5579 (2007).
Satterfield, C. N., Colton, C. K. & Pitcher, W. H.Jr Restricted diffusion in liquids within fine pores. AIChE J. 19, 628–635 (1973).
Sané, J., Padding, J. T. & Louis, A. A. The crossover from single file to Fickian diffusion. Faraday Discuss. 144, 285–299 (2010).
Cummings, P. T., Docherty, H., Iacovella, C. R. & Singh, J. K. Phase transitions in nanoconfined fluids: the evidence from simulation and theory. AIChE J. 56, 842–848 (2010).
Merlet, C. et al. Highly confined ions store charge more efficiently in supercapacitors. Nat. Commun. 4, 2701 (2013).
Laziz, N. A. et al. Li-and Na-ion storage performance of natural graphite via simple flotation process. J. Electrochem. Sci. Technol. 9, 320–329 (2018).
Li, S. et al. Intercalation of metal ions into Ti3C2Tx MXene electrodes for high-areal-capacitance microsupercapacitors with neutral multivalent electrolytes. Adv. Funct. Mater. 30, 2003721 (2020).
Li, Y. et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 19, 894–899 (2020).
Zukalová, M., Procházka, J., Lásková, B. P., Zukal, A. & Kavan, L. Layered LiNi1/3Mn1/3Co1/3O2 (NMC) with optimized morphology for Li-ion batteries. ECS Trans. 87, 67–75 (2018).
Acknowledgements
P.T.C., J.W., Y.G. and V.A. acknowledge support from the Fluid Interface Reactions, Structures and Transport (FIRST) Center, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Office of Basic Energy Sciences at Oak Ridge National Laboratory under contract no. DE-AC0500OR22725 with UT Battelle, LLC. S.F. acknowledges funding from the German Federal Ministry of Education and Research (BMBF) in the ‘NanoMatFutur’ program (grant no. 03XP0423). S.F. and P.S. acknowledge support from the Agence Nationale de la Recherche (Labex STORE-EX) and P.S. from the ERC Synergy Grant MoMa-Stor no. 951513.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Christophe Lethien, Xingbin Yan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fleischmann, S., Zhang, Y., Wang, X. et al. Continuous transition from double-layer to Faradaic charge storage in confined electrolytes. Nat Energy 7, 222–228 (2022). https://doi.org/10.1038/s41560-022-00993-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-022-00993-z
This article is cited by
-
Cation desolvation-induced capacitance enhancement in reduced graphene oxide (rGO)
Nature Communications (2024)
-
Capacitive tendency concept alongside supervised machine-learning toward classifying electrochemical behavior of battery and pseudocapacitor materials
Nature Communications (2024)
-
Dielectric polymer grafted electrodes enhanced aqueous supercapacitors
Nano Research (2024)
-
Electrochemically modulated interaction of MXenes with microwaves
Nature Nanotechnology (2023)
-
In situ monitoring redox processes in energy storage using UV–Vis spectroscopy
Nature Energy (2023)