Macroplastic Storage and Remobilization in Rivers
Abstract
:1. Introduction
2. Plastic as a New, Artificial Type of River Load
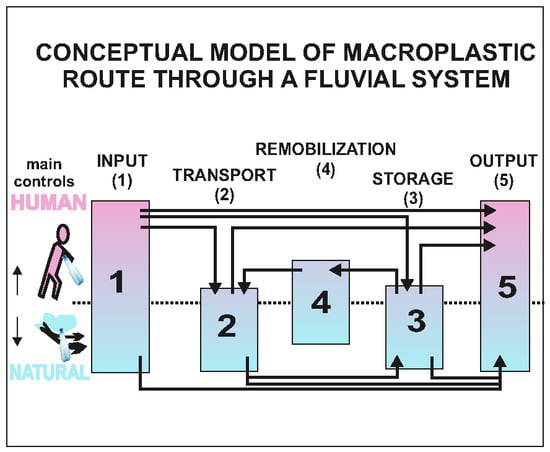
3. Conceptual Model of Macroplastic Routing through a Fluvial System
3.1. Input of Macroplastic to Fluvial System
3.2. Macroplastic Transport
3.3. Macroplastic Storage
3.4. Macroplastic Remobilization
3.5. Macroplastic Output
4. Perspectives on Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zalasiewicz, J.; Waters, C.N.; Sul, J.I.D.; Corcoran, P.L.; Yonan, Y. The geological cycle of plastics and their use as a stratigraphic indicator of the Anthropocene. Anthropocene 2016, 13, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B 2019, 364, 1985–1998. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; vom Saal, F.S. Our plastic age. Philos. Trans. R. Soc. B 2009, 364, 1973–1976. [Google Scholar] [CrossRef] [Green Version]
- Zalasiewicz, J.; Gabbott, S.; Waters, C.N. Plastic waste: How plastics have become part of the Earth’s geological cycle. In Waste: A Handbook for Management; Letcher, T.M., Vallero, D.A., Eds.; Academic Press: London, UK, 2019; pp. 443–451. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, G.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Besseling, E.; Foekema, E.; Kooi, M.; Mintenig, S.; Ossendorp, B.C.; Redondo-Hasselerharm, P.E.; Verschoor, A.; van Wezel, A.P.; Scheffer, M.; et al. Risks of plastic debris: Unravelling fact, opinion, perception, and belief. Environ. Sci. Technol. 2017, 51, 11513–11519. [Google Scholar] [CrossRef]
- Chae, Y.; An, Y.-J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Rist, S.; Almroth, B.C.; Hartmann, N.B.; Karlsson, T.M. A critical perspective on early communications concerning human health aspects of microplastics. Sci. Total Environ. 2018, 626, 720–726. [Google Scholar] [CrossRef] [Green Version]
- Bank, M.S.; Hansson, S.V. The plastic cycle: A novel and holistic paradigm for the Anthropocene. Environ. Sci. Technol. 2019, 53, 7177–7179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blettler, M.C.; Wantzen, K.M. Threats underestimated in freshwater plastic pollution: Mini-review. Water Air Soil Pollut. 2019, 230, 174. [Google Scholar] [CrossRef]
- Hurley, R.; Horton, A.; Lusher, A.; Nizzetto, L. Plastic waste in the terrestrial environment. Plast. Waste Recycl. 2020, 163–193. [Google Scholar] [CrossRef]
- van Emmerik, T.; Schwarz, A. Plastic debris in rivers. WIREs. Water 2020, 7, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Waldschläger, K.; Lechthaler, S.; Stauch, G.; Schüttrumpf, H. The way of microplastic through the environment—Application of the source–pathway–receptor model (review). Sci. Total Environ. 2020, 713, 136584. [Google Scholar] [CrossRef] [PubMed]
- Winton, D.J.; Anderson, L.G.; Rocliffe, S.; Loiselle, S. Macroplastic pollution in freshwater environments: Focusing public and policy action. Sci. Total Environ. 2020, 704, 135242. [Google Scholar] [CrossRef] [PubMed]
- Blettler, M.C.; Abrial, E.; Khan, F.R.; Sivri, N.; Espinola, L.A. Freshwater plastic pollution: Recognizing research biases and identifying knowledge gaps. Water Res. 2018, 143, 416–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, C.; Krauth, T.; Wagner, S. Export of plastic debris by rivers into the sea. Environ. Sci. Technol. 2017, 51, 12246–12253. [Google Scholar] [CrossRef]
- van Calcar, C.J.; van Emmerik, T. Abundance of plastic debris across European and Asian rivers. Environ. Res. Lett. 2019, 14, 124051. [Google Scholar] [CrossRef] [Green Version]
- Gabbott, S.; Russell, C.; Yohan, Y.; Zalasiewicz, J. The geography and geology of plastics: Their environmental distribution and fate. Plast. Waste Recycl. 2020, 33–63. [Google Scholar] [CrossRef]
- He, B.; Goonetilleke, A.; Ayoko, G.A.; Rintoul, L. Abundance, distribution patterns, and identification of microplastics in Brisbane River sediments, Australia. Sci. Total Environ. 2020, 700, 134467. [Google Scholar] [CrossRef]
- Tramoy, R.; Gasperi, J.; Colasse, L.; Tassin, B. Transfer dynamic of macroplastics in estuaries—New insights from the Seine estuary: Part 1. Long term dynamic based on date-prints on stranded debris. Mar. Pollut. Bull. 2020, 152, 110894. [Google Scholar] [CrossRef]
- Horton, A.A.; Dixon, S.J. Microplastic: An introduction to environmental transport processes. WIREs. Water 2017, 5, e1268. [Google Scholar] [CrossRef] [Green Version]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018, 11, 251–257. [Google Scholar] [CrossRef]
- Wagner, S.; Klockner, P.; Stier, B.; Romer, M.; Seiwert, B.; Reemtsma, T.; Schmidt, C. Relationship between discharge and river plastic concentrations in a rural and an urban catchment. Environ. Sci. Technol. 2019, 53, 10082–10091. [Google Scholar] [CrossRef] [PubMed]
- Windsor, F.M.; Durance, I.; Horton, A.A.; Thompson, R.C.; Tyler, C.R.; Ormerod, S.J. A catchment-scale perspective of plastic pollution. Glob. Chang. Biol. 2019, 25, 1207–1221. [Google Scholar] [CrossRef] [Green Version]
- van Emmerik, T.; Strady, E.; Kieu-Le, T.; Nguyen, L.; Gratiot, N. Seasonality of riverine macroplastic transport. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- van Emmerik, T.; Tramoy, R.; van Calcar, C.; Alligant, S.; Treilles, R.; Tassin, B.; Gasperi, J. Seine plastic debris transport tenfolded during increased river discharge. Front. Mar. Sci. 2019, 6, 642. [Google Scholar] [CrossRef]
- Guerranti, C.; Perra, G.; Martellini, T.; Giari, L.; Cincinelli, A. Knowledge about microplastic in Mediterranean tributary river ecosystems: Lack of data and research needs on such a crucial marine pollution source. J. Mar. Sci. Eng. 2020, 8, 216. [Google Scholar] [CrossRef] [Green Version]
- Nihei, Y.; Yoshida, T.; Kataoka, T.; Ogata, R. High-resolution mapping of Japanese microplastic and macroplastic emissions from the land into the sea. Water 2020, 12, 951. [Google Scholar] [CrossRef] [Green Version]
- Cozzolino, L.; Nicastro, K.R.; Zardi, G.I.; de los Santos, C.B. Species-specific plastic accumulation in the sediment and canopy of coastal vegetated habitats. Sci. Total Environ. 2020, 723, 138018. [Google Scholar] [CrossRef]
- Bonney, R.; Shirk, J.L.; Phillips, T.B.; Wiggins, A.; Ballard, H.L.; Miller-Rushing, A.J.; Parrish, J.K. Next steps for citizen science. Science 2014, 243, 1427–1436. [Google Scholar] [CrossRef]
- Heidbreder, M.; Bablok, I.; Drews, S.; Menzel, C. Tackling the plastic problem: A review on perceptions, behaviours, and interventions. Sci. Total Environ. 2019, 668, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.; Green, C. Making sense of microplastics? Public understandings of plastic pollution. Mar. Pollut. Bull. 2020, 152, 110908. [Google Scholar] [CrossRef] [PubMed]
- Lavers, J.L.; Bond, A.L. Exceptional and rapid accumulation of anthropogenic debris on one of the world’s most remote and pristine islands. Proc. Natl. Acad. Sci. USA 2017, 114, 6052–6055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geraeds, M.; van Emmerik, T.; de Vries, R.; bin Ab Razak, M.S. Riverine plastic litter monitoring using unmanned aerial vehicles (UAVs). Remote Sens. 2019, 11, 2045. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Vicente, V.; Clark, J.R.; Corradi, P.; Aliani, S.; Arias, M.; Bochow, M.; Bonnery, G.; Cole, M.; Cózar, A.; Donnelly, R.; et al. Measuring marine plastic debris from space: Initial assessment of observation requirements. Remote Sens. 2019, 11, 2443. [Google Scholar] [CrossRef] [Green Version]
- Andriolo, U.; Gonçalves, G.; Bessa, F.; Sobral, P. Mapping marine litter on coastal dunes with unmanned aerial systems: A showcase on the Atlantic Coast. Sci. Total Environ. 2020, 736, 139632. [Google Scholar] [CrossRef]
- Biermann, L.; Clewley, D.; Martinez-Vicente, V.; Topouzelis, K. Finding plastic patches in coastal waters using optical satellite data. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Gonçalves, G.; Andriolo, U.; Pinto, L.; Duarte, D. Mapping marine litter with unmanned aerial systems: A showcase comparison among manual image screening and machine learning techniques. Mar. Pollut. Bull. 2020, 155, 111158. [Google Scholar] [CrossRef]
- Kataoka, T.; Nihei, Y. Quantification of floating riverine macro-debris transport using an image processing approach. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Merlino, S.; Paterni, M.; Berton, A.; Massetti, L. Unmanned aerial vehicles for debris survey in coastal areas: Long-term monitoring programme to study spatial and temporal accumulation of the dynamics of beached marine litter. Remote Sens. 2020, 12, 1260. [Google Scholar] [CrossRef] [Green Version]
- Leopold, L.B.; Wolman, M.G.; Miller, J.P. Fluvial Processes in Geomorphology; Freeman: San Francisco, CA, USA, 1964; p. 522. ISBN 978-0486685885. [Google Scholar]
- Gurnell, A.M.; Piégay, H.; Swanson, F.J.; Gregory, S.V. Large wood and fluvial processes. Freshw. Biol. 2002, 47, 601–619. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Villanueva, V.; Piégay, H.; Gurnell, A.M.; Marston, R.A.; Stoffel, M. Recent advances quantifying the large wood dynamics in river basins: New methods and remaining challenges. Rev. Geophys. 2016, 54, 611–652. [Google Scholar] [CrossRef] [Green Version]
- Shumilova, O.; Tockner, K.; Gurnell, A.M.; Langhans, S.D.; Righetti, M.; Lucía, A.; Zarfl, C. Floating matter: A neglected component of the ecological integrity of rivers. Aquat. Sci. 2019, 81, 25. [Google Scholar] [CrossRef]
- Hoellein, T.J.; Shogren, A.J.; Tank, J.L.; Risteca, P.; Kelly, J.J. Microplastic deposition velocity in streams follows patterns for naturally occurring allochthonous particles. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ockelford, A.; Cundy, A.; Ebdon, J.E. Storm response of fluvial sedimentary microplastics. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Bruge, A.; Barreau, C.; Carlot, J.; Collin, H.; Moreno, C.; Maison, P. Monitoring litter inputs from the Adour River (Southwest France) to the marine environment. J. Mar. Sci. Eng. 2018, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Wohl, E.; Kramer, N.; Ruiz-Villanueva, V.; Scott, D.N.; Comiti, F.; Gurnell, A.M.; Piégay, H.; Lininger, K.B.; Jaeger, K.L.; Walters, D.M.; et al. The natural wood regime in rivers. BioScience 2019, 69, 259–273. [Google Scholar] [CrossRef]
- Tramoy, R.; Gasperi, J.; Dris, R.; Colasse, L.; Fisson, C.; Sananes, S.; Rocher, V.; Tassin, B. Assessment of the plastic inputs from the Seine basin to the sea using statistical and field approaches. Front. Mar. Sci. 2019, 6, 151. [Google Scholar] [CrossRef] [Green Version]
- Weideman, E.A.; Perold, V.; Ryan, P.G. Limited long-distance transport of plastic pollution by the Orange-Vaal River system, South Africa. Sci. Total Environ. 2020, 727, 138653. [Google Scholar] [CrossRef]
- Malinowski, M.; Wolny-Koładka, K.; Jastrzębski, B. Characteristics of illegal dumping sites—Case study: Watercourses. Infrastrukt. Ekol. Teren. Wiej. 2015, 4, 1475–1484. [Google Scholar] [CrossRef]
- Castro-Jiménez, J.; González-Fernández, D.; Fornier, M.; Schmidt, N.; Sempere, R. Macro-litter in surface waters from the Rhone River: Plastic pollution and loading to the NW Mediterranean Sea. Mar. Pollut. Bull. 2019, 146, 60–66. [Google Scholar] [CrossRef]
- Ciszewski, D.; Grygar, T.M. A Review of flood-related storage and remobilization of heavy metal pollutants in river systems. Water Air Soil Pollut. 2016, 227, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Wijnen, J.A.; Ragas, M.J.; Kroeze, C. Modelling global river export of microplastics to the marine environment: Sources and future trends. Sci. Total Environ. 2019, 673, 392–401. [Google Scholar] [CrossRef] [PubMed]
- van Emmerik, T.; Kieu-Le, T.-C.; Loozen, M.; van Oeveren, K.; Strady, E.; Bui, X.-T.; Egger, M.; Gasperi, J.; Lebreton, L.; Nguyen, P.-D.; et al. A methodology to characterize riverine macroplastic emission into the ocean. Front. Mar. Sci. 2018, 5, 372. [Google Scholar] [CrossRef] [Green Version]
- van Emmerik, T.; Loozen, M.; Van Oeveren, K.; Buschman, F.; Prinsen, G. Riverine plastic emission from Jakarta into the ocean. Environ. Res. Lett. 2019, 14, 08403. [Google Scholar] [CrossRef] [Green Version]
- Wyżga, B.; Mikuś, P.; Zawiejska, J.; Ruiz-Villanueva, V.; Kaczka, R.J.; Czech, W. Log transport and deposition in incised, channelized, and multithread reaches of a wide mountain river: Tracking experiment during a 20-year flood. Geomorphology 2017, 279, 98–111. [Google Scholar] [CrossRef]
- Vriend, P.; van Emmerik, T.; Roebroek, C.T.J. Same but different: A framework to design and compare riverbank plastic monitoring strategies. Earth Ar Xiv 2020. [Google Scholar] [CrossRef]
- Kiss, T.; Nagy, J.; Fehérváry, I.; Vaszkó, C. (Mis) management of floodplain vegetation: The effect of invasive species on vegetation roughness and flood levels. Sci. Total Environ. 2019, 686, 931–945. [Google Scholar] [CrossRef]
- Liro, M. Dam reservoir backwater as a field-scale laboratory of human-induced changes in river biogeomorphology: A review focused on gravel-bed rivers. Sci. Total Environ. 2019, 651, 2899–2912. [Google Scholar] [CrossRef]
- Blettler, M.C.; Garello, N.; Ginon, L.; Abrial, E.; Espinola, L.A.; Wantzen, K.M. Massive plastic pollution in a mega-river of developing country: Sediment deposition and ingestion by fish (Prochilodus lineatus). Environ. Poll. 2019, 225, 113348. [Google Scholar] [CrossRef]
- Dalu, T.; Malesa, B.; Cuthbert, R.N. Assessing factors driving the distribution and characteristics of shoreline macroplastics in a subtropical reservoir. Sci. Total Environ. 2019, 696, 133992. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, T.; Knickmeier, K.; Kruse, K.; Brennecke, D.; Nauendorf, A.; Thiel, M. Plastic Pirates sample litter at rivers in Germany—Riverside litter and litter sources estimated by schoolchildren. Environ. Pollut. 2019, 245, 545–557. [Google Scholar] [CrossRef]
- Hoellein, T.J.; Rojas, M.; Pink, A.; Gasior, J.; Kelly, J. Anthropogenic litter in urban freshwater ecosystems: Distribution and microbial interactions. PLoS ONE 2014, 9, e98485. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.J.; Das Sarkar, S.; Das, B.K.; Manna, R.K.; Behera, B.J.; Srikanta, S. Spatial distribution of meso and microplastics in the sediments of river Ganga at eastern India. Sci. Total Environ. 2019, 694, 133712. [Google Scholar] [CrossRef] [PubMed]
- Gurnell, A.; Thompson, K.; Goodson, J.; Moggridge, H. Propagule deposition along river margins: Linking hydrology and ecology. J. Ecol. 2008, 96, 553–565. [Google Scholar] [CrossRef]
- Tramoy, R.; Colasse, L.; Gasperi, J.; Tassin, B. Plastic debris dataset on the Seine river banks: Plastic pellets, unidentified plastic fragments and plastic sticks are the Top 3 items in a historical accumulation of plastics. Data Brief 2019, 23, 103697. [Google Scholar] [CrossRef]
- Acha, E.M.; Mianzan, H.W.; Iribarne, O.; Gagliardini, D.A.; Lasta, C.; Daleo, P. The role of the Rıo de la Plata bottom salinity front in accumulating debris. Mar. Pollut. Bull. 2003, 46, 197–202. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef]
- Ghaffari, S.; Bakhtiari, A.R.; Ghasempouri, S.M.; Nasrolahi, A. The influence of human activity and morphological characteristics of beaches on plastic debris distribution along the Caspian Sea as a closed water body. Environ. Sci. Pollut. Res. 2019, 26, 25712–25724. [Google Scholar] [CrossRef]


| Reference | Habitat Type, Sampling Description | Results Unit |
|---|---|---|
| [69] | Lake shoreline. Surface sediments. Hand collection of all artificial litter (including plastic) along variable-width zone of 7.8 km (2% of lake shoreline). | g/km % of items type |
| [63] | Reservoir shoreline survey (2 replicates, 2 seasons), hand collection of macroplastic debris (>2.5 cm) within 5 × 5 m quadrats located from 0 to 50 m from shoreline. | items/site g/site |
| [65] | Riverbank and channel zone. Surface sediment: hand collection of all anthropogenic litter (including plastic) along riverbank within 10-m-wide zone of water edge and in river channel along three 70–100 m reaches. Lake beaches. Surface sediments: hand collection along three 400 × 50 m transects of shoreline (3-day surveys). | items/m2 g/m2 surface area (cm2)/m2 |
| [48] | Riverbanks and floodplain sampled monthly (during the years 2014–2017) along whole river course from source to estuary. Surface sediments: hand collection of all artificial litter (including plastic) (>5 mm) within sites selected along whole fluvial system. | items/site kg/site types (%) of items/site items/collection/100 m |
| [62] | Riverbank. Surface and subsurface sediments. Hand collection of macroplastic (>2.5 cm) within two, randomly chosen transects (3 × 50 m) selected parallel to the riverbank and covering more than 20% of shoreline section. Mesoplastic (0.5–2.5 cm) collection by sieving (using stainless sieves of 5 mm mesh size) of the top 3 cm layer of sediments within three 1 m2 quadrats, randomly located in each macroplastic transect, following the macroplastic collection. | items/transect items/m2 |
| [35] | River channel and bank. Flowing water and surface sediments. Identification of macroplastic (>2 cm) from aerial photographs done using UAV (DJI Phantom 4) from the altitude of 5 and 15 m. | items/m2 |
| [64] | Riverbank and floodplain. Surface sediments: hand collection of all artificial litter (including plastic) within three sampling sites (circle of radius 1.5 m, ~7 m2) located within river edge (0–5 m from channel), riverbank (5–15 m) and river crest (>15 m) along the transect perpendicular to river course (up to three transects per site) | items/m2 surface area of items (%, m2)/m2 or/site |
| [66] | Riverbank. Surface and subsurface sediments. Hand collection (steel spoon) of 2–3 kg samples within the area of 15–20 cm2, followed by sieve analysis (mesh sizes: 10 mm, 5 mm, 850 μm, 63 μm) and density separation in ZnCl2 solution. | ng/g (mass fraction) items/kg |
| [70] | Riverbank (river marsh), surface sediments: hand collection of macro (>2.5 cm) and mesoplastic debris (0.5–2.5 cm). | items/m2 g/m2 g/item type % of items type/total items |
| [30] | Tidal zone, habitats of different vegetation types. Emergent and underwater surface sediments: hand collection of macroplastic debris (>0.5 cm) within three 5 × 5 m quadrats per plot (surface sediments in intertidal zone) and three underwater band transects 6 × 4 m (subtidal zone). Macroplastic debris size classified at 10-cm intervals. | items/100 m2 kg/100 m2 % of items/habitat type |
| [21] | River estuary (old dredging chamber, active floodplain, floodplain part disconnected by artificial levee). Surface sediments: targeted collection of microlax packaging items with printed use-by dates. | types and number of plastic debris with date-prints items (microlax)/site |
| [34] | Marine beach, surface sediments: hand and sieve collection of macroplastic debris (>2 mm and >5 mm) within five 5 × 30 m and ten 20 × 2 m transects. Subsurface sediments (0–10 cm): sieve collection of macroplastic debris in ten 0.4 × 0.4 m quadrats. Monitoring of plastic debris accumulation along 10 × 0.2 m transect (six days). Total amount of debris calculated as mean surface/subsurface densities per site area. | items/m2 kg/site |
| [71] | Marine beach. Surface and subsurface sediments. Hand collection and sieving of top 5 cm layer of sediments within 18 quadrats 1 m2 in size located at equal distances of 5 m along three transects parallel to beach (repeated twice at the same location). | items/m2 maximum linear length of plastic litter |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liro, M.; Emmerik, T.v.; Wyżga, B.; Liro, J.; Mikuś, P. Macroplastic Storage and Remobilization in Rivers. Water 2020, 12, 2055. https://doi.org/10.3390/w12072055
Liro M, Emmerik Tv, Wyżga B, Liro J, Mikuś P. Macroplastic Storage and Remobilization in Rivers. Water. 2020; 12(7):2055. https://doi.org/10.3390/w12072055
Chicago/Turabian StyleLiro, Maciej, Tim van Emmerik, Bartłomiej Wyżga, Justyna Liro, and Paweł Mikuś. 2020. "Macroplastic Storage and Remobilization in Rivers" Water 12, no. 7: 2055. https://doi.org/10.3390/w12072055