Algae-Laden Fouling Control by Gravity-Driven Membrane Ultrafiltration with Aluminum Sulfate-Chitosan: The Property of Floc and Cake Layer
Abstract
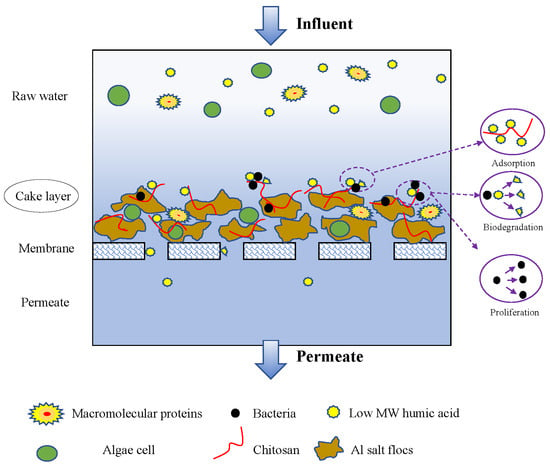
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Raw Water
2.2. Preparation of Coagulant
2.3. Experimental Set-up
2.3.1. Coagulation/GDM System
2.3.2. UF Membrane Module
2.4. Ultrafiltration Process
2.4.1. Filtration Flux and Membrane Fouling Resistance
2.4.2. Modeling for Membrane Fouling Process
2.5. Floc Property
2.6. Other Analytical Methods
3. Results
3.1. Membrane Filtration Performance
3.1.1. Membrane Permeate Flux and Fouling Resistance
3.1.2. Membrane Fouling Mechanism
3.2. Filtration Permeate Quality
3.2.1. DOC Concentration
3.2.2. Characterization of Organic Matter
3.2.3. Biomass Concentration
4. Discussions
5. Conclusions
- Compared with AS/GDM and the GDM control system, the AS-CS/GDM system alleviated 26.40% and 53.24% of hydraulically reversible resistance, and 14.89% and 68.33% of hydraulically irreversible resistance, respectively. The application of AS-CS pre-coagulation can prolong the life of the GDM membrane.
- Compared with the AS/GDM system, the AS-CS/GDM system effectively removed humic-like substances in the MW range of 3 to 100 kDa, while slightly improving the protein-like substance, resulting in a 7.53% lower concentration of DOC.
- The presence of AS-CS pre-coagulation increased the concentration of attached biomass, which improved the biodegradability of microorganisms attached on the UF membrane surface.
- The generation of larger flocs in the AS-CS pre-coagulation step led to a porous cake layer with light Rcl. The presence of the CS polymeric chain in the AS-CS cake layer, together with humic-like substances, can form small aggregations, which were suitable for the proliferation of microorganisms with more attached biomass. The microorganisms effectively remove humic-like substances in the MW range of 3 to 100 kDa, resulting in the mitigation of pore blockage.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, B.; Qu, F.S.; Liang, H.; Van der Bruggen, B.; Cheng, X.X.; Yu, H.R.; Xu, G.R.; Li, G.B. Microcystis aeruginosa-laden surface water treatment using ultrafiltration: Membrane fouling, cell integrity and extracellular organic matter rejection. Water Res. 2017, 112, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.S.; Du, X.; Liu, B.; He, J.G.; Ren, N.Q.; Li, G.B.; Liang, H. Control of ultrafiltration membrane fouling caused by Microcystis cells with permanganate preoxidation: Significance of in situ formed manganese dioxide. Chem. Eng. J. 2015, 279, 56–65. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Shao, Y.S.; Gao, N.Y.; Deng, Y.; Li, L.; Deng, J.; Tan, C.Q. Characterization of algal organic matters of Microcystis aeruginosa: Biodegradability, DBP formation and membrane fouling potential. Water Res. 2014, 52, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, Q. Algal fouling of microfiltration and ultrafiltration membranes and control strategies: A review. Sep. Purif. Technol. 2018, 203, 193–208. [Google Scholar] [CrossRef]
- Yu, C.H.; Gao, B.Y.; Wang, W.Y.; Xu, X.; Yue, Q.Y. Alleviating membrane fouling of modified polysulfone membrane via coagulation pretreatment/ultrafiltration hybrid process. Chemosphere 2019, 235, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.; Szczuka, A.; Shabani, F.; Munoz, J.; Aflaki, R.; Hammond, S.; Mitch, W. Pilot-scale comparison of microfiltration/reverse osmosis and ozone/biological activated carbon with UV/hydrogen proxide or UV/free chlorine AOP treatment for controlling disinfection byproducts during wastewater reuse. Water Res. 2019, 152, 215–225. [Google Scholar] [CrossRef]
- Chang, H.Q.; Liu, B.C.; Wang, H.Z.; Zhang, S.; Chen, S.; Tiraferri, A.; Tang, Y. Evaluating the performance of gravity-driven membrane filtration as desalination pretreatment of shale gas flowback and produced water. J. Membr. Sci. 2019, 587, 117187. [Google Scholar] [CrossRef]
- Tang, X.B.; Pronk, W.; Ding, A.; Cheng, X.X.; Wang, J.L.; Xie, B.H.; Li, G.B.; Liang, H. Coupling GAC to ultra-low-pressure filtration to modify the biofouling layer and bio-community: Flux enhancement and water quality improvement. Chem. Eng. J. 2018, 333, 289–299. [Google Scholar] [CrossRef]
- Ding, A.; Liang, H.; Li, G.B.; Derlon, N.; Szivak, I.; Morgenroth, E.; Pronk, W. Impact of aeration shear stress on permeate flux and fouling layer properties in a low pressure membrane bioreactor for the treatment of grey water. J. Membr. Sci. 2016, 510, 382–390. [Google Scholar] [CrossRef]
- Peter-Varbanets, M.; Margot, J.; Traber, J.; Pronk, W. Mechanism of membrane fouling during ultra-low pressure ultrafiltration. J. Membr. Sci. 2011, 377, 42–53. [Google Scholar] [CrossRef]
- Derlon, N.; Mimoso, J.; Klein, T.; Koetzsch, S.; Morgenroth, E. Presence of biofilms on ultrafiltration membrane surfaces increases the quality of permeate produced during ultra-low pressure gravity-driven membrane filtration. Water Res. 2014, 60, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Chomiak, A.; Traber, J.; Morgenroth, E.; Derlon, N. Biofilm increases permeate quality by organic carbon degradation in low pressure ultrafiltration. Water Res. 2015, 85, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Peter-Varbanets, M.; Gujer, W.; Pronk, W. Intermittent operation of ultra-low pressure ultrafiltration for decentralized drinking water treatment. Water Res. 2012, 46, 3272–3282. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Wang, J.; Lin, D.; Tang, X.; Cheng, X.X.; Wang, H.; Bai, L.; Li, G.B.; Liang, H. A low pressure gravity-driven membrane filtration (GDM) system for rainwater recycling: Flux stabilization and removal performance. Chemosphere 2017, 172, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Akhondi, E.; Wu, B.; Sun, S.; Marxer, B.; Lim, W.; Gu, J.; Liu, L.; Burkhardt, M.; McDougald, D.; Pronk, W.; et al. Gravity-driven membrane filtration as pre-treatment for seawater reverse osmosis: Linking biofouling layer morphology with flux stabilization. Water Res. 2015, 70, 158–173. [Google Scholar] [CrossRef]
- Yu, W.Z.; Liu, T.; Gregory, J.; Campos, L.; Li, G.B.; Qu, J.H. Influence of flocs breakage process on submerged ultrafiltration membrane fouling. J. Membr. Sci. 2011, 385–386, 194–199. [Google Scholar] [CrossRef]
- Ding, A.; Wang, J.L.; Lin, D.C.; Tang, X.B.; Cheng, X.X.; Li, G.B.; Ren, N.Q.; Liang, H. In situ coagulation versus pre-coagulation for gravity-driven membrane bioreactor during decentralized sewage treatment: Permeability stabilization, fouling layer formation and biological activity. Water Res. 2017, 126, 197–207. [Google Scholar] [CrossRef]
- Qi, J.; Lan, H.C.; Liu, R.P.; Liu, H.J.; Qu, J.H. Fe(II)-regulated moderate pre-oxidation of Microcystis aeruginosa and formation of size-controlled algae flocs for efficient flotation of algae cell and organic matter. Water Res. 2018, 137, 57–63. [Google Scholar] [CrossRef]
- Ji, J.; Qiu, J.; Wai, N.; Wong, F.S.; Li, Y. Influence of organic and inorganic flocculants on physical-chemical properties of biomass and membrane-fouling rate. Water Res. 2010, 44, 1627–1635. [Google Scholar] [CrossRef]
- Ma, C.X.; Hu, W.R.; Pei, H.Y.; Xu, H.Z.; Pei, R.T. Enhancing integrated removal of Microcystis aeruginosa and adsorption of Microcystins using chitosan-aluminum chloride combined coagulants: Effect of chemical dosing orders and coagulation mechanisms. Colloid Surf. A 2016, 490, 258–267. [Google Scholar] [CrossRef]
- Wang, W.Y.; Qu, F.S.; Li, R.H.; Bu, F.; Shen, X.; Gao, B.Y. Optimization of coagulation pre-treatment for alleviating ultrafiltration membrane fouling: The role of floc properties on Al species. Chemosphere 2018, 200, 86–92. [Google Scholar] [CrossRef]
- Cheng, X.X.; Liang, H.; Ding, A.; Zhu, X.W.; Tang, X.B.; Gan, Z.D.; Xing, J.J.; Wu, D.J.; Li, G.B. Application of Fe(II)/peroxymonosulfate for improving ultrafiltration membrane performance in surface water treatment: Comparison with coagulation and ozonation. Water Res. 2017, 124, 298–307. [Google Scholar] [CrossRef]
- Choo, K.H.; Lee, C.H. Membrane fouling mechanisms in the membrane-coupled anaerobic bioreactor. Water Res. 1996, 30, 1771–1780. [Google Scholar] [CrossRef]
- Yu, W.Z.; Zhang, D.J.; Graham, N. Membrane fouling by extracellular polymeric substances after ozone pre-treatment: Variation of nano-particles size. Water Res. 2017, 120, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Hermia, J. Constant pressure blocking filtration laws-appllication to power-law non-Newtonian fluids. Trans. Inst. Chem. Eng. 1982, 60, 183–187. [Google Scholar]
- Bowen, W.R.; Calvo, J.I.; Hernadez, A. Steps of membrane blocking in flux decline during protein microfiltration. J. Member. Sci. 1995, 101, 153–165. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Yang, Y.L.; Li, X.; Wang, W.Q.; Wu, Y.; Wang, C.Y.; Luo, J.L. Coagulation performance and flocs characteristics of recycling pre-sonicated condensate sludge for low-turbidity surface water treatment. Sep. Purif. Technol. 2014, 123, 1–8. [Google Scholar] [CrossRef]
- Wang, Z.B.; Nan, J.; Yao, M.; Yang, Y.M.; Zhang, P.Y. Insight into the combined coagulation-ultrafiltration process: The role of Al species of polyaluminum chlorides. Rsc Adv. 2017, 6, 48745–48752. [Google Scholar] [CrossRef]
- Servais, P.; Anzil, A.; Ventresque, C. Simple method for determination of biodegradable dissolved organic carbon in water. Appl. Environ. Microb. 1989, 55, 2732–2734. [Google Scholar] [CrossRef] [Green Version]
- Bu, F.; Gao, B.Y.; Li, R.H.; Sun, S.L.; Yue, Q.Y. Impacts of epichlorohydrin-dimethylamine on coagulation performance and membrane fouling in coagulation/ultrafiltration combined process with different Al-based coagulants. Chemosphere 2016, 159, 228–234. [Google Scholar] [CrossRef]
- Ma, B.W.; Wang, X.; Hu, C.Z.; Jefferson, W.A.; Liu, H.J.; Qu, J.H. Antifouling by pre-deposited Al hydrolytic flocs on ultrafiltration membrane in the presence of humic acid and bovine serum albumin. J. Member. Sci. 2017, 538, 34–40. [Google Scholar] [CrossRef]
- Sun, S.L.; Gao, B.Y.; Yue, Q.Y.; Li, R.H.; Song, W.; Bu, F.; Zhao, S.; Jia, R.B.; Song, W.C. Comparison of epichlorohydrin-dimethylamine with other cationic organic polymers as coagulation aids of polyferric chloride in coagulation-ultrafiltration process. J. Hazard. Mater. 2016, 307, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Z.M.; Rietveld, L.C.; Gao, N.Y.; Hu, J.Y.; Yin, D.Q.; Yu, S.L. Comparison of the effects of extracellular and intracellular organic matter extracted from Microcystis aeruginosa on ultrafiltration membrane fouling: Dynamics and mechanisms. Environ. Sci. Technol. 2014, 48, 14549–14557. [Google Scholar] [CrossRef]
- Tian, J.Y.; Chen, Z.L.; Yang, Y.L.; Liang, H.; Nan, J.; Wang, Z.Z.; Li, G.B. Hybrid process of BAC and sMBR for treating polluted raw water. Bioresour. Technol. 2009, 100, 6243–6249. [Google Scholar] [CrossRef]
- Ayech, A.; Josende, M.E.; Ventura-Lima, J.; Ruas, C.; Gelesky, M.A.; Ale, A.; Cazenave, J.; Galdoporpora, J.M.; Desimone, M.F.; Duarte, M.; et al. Toxicity evaluation of nanocrystalline silver-impregnated coated dressing on the life cycle of worm Caenorhabditis elegans. Ecotox. Environ. Safe. 2020, 197, 110570. [Google Scholar] [CrossRef]
- Wang, H.S.; Feng, C.P.; Deng, Y. Effect of potassium on nitrate removal from groundwater in agricultural waste-based heterotrophic denitrification system. Sci. Total Environ. 2020, 703, 134830. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Q.; Gao, L.; Gu, J.Y.; Zhou, W.L.; Fan, C.Z.; He, S.B.; Huang, J.C.; Zhang, X.; Cheng, Y.F.; Wu, Z.W.; et al. Enhancement of nitrogen removal via addition of cattail litter in surface flow constructed wetland. J. Clean. Prod. 2018, 204, 205–211. [Google Scholar] [CrossRef]
- Shen, X.; Gao, B.Y.; Guo, K.Y.; Yue, Q.Y. Characterization and influence of floc under different coagulation system on ultrafiltration membrane fouling. Chemosphere 2020, 238, 124659. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Yue, Q.Y.; Li, R.H.; Song, W.; Gao, B.Y.; Shen, X. Investigating coagulation behavior of chitosan with different Al species dual-coagulants in dye wastewater treatment. J. Taiwan Inst. Chem. Eng. 2017, 78, 423–430. [Google Scholar] [CrossRef]
- Du, H.W.; Yang, Z.; Tian, Z.Q.; Huang, M.L.; Yang, W.B.; Zhang, L.M.; Li, A.M. Enhanced removal of trace antibiotics from turbid water in the coexistence of natural organic matters using phenylalanine-modified-chitosan flocculants: Effect of flocculants’ molecular architectures. Chem. Eng. J. 2018, 333, 310–319. [Google Scholar] [CrossRef]
- Ma, B.W.; Li, W.J.; Liu, R.P.; Liu, G.; Sun, J.Q.; Liu, H.J.; Qu, J.H.; van der Meer, W. Multiple dynamic Al-based floc layers on ultrafiltration membrane surfaces for humic acid and reservoir water fouling reduction. Water Res. 2018, 139, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, J.P.; Chen, H.F.; Yin, X.H.; Wang, N. Structural Characteristics of Humic-like Acid from Microbial Transformation of Lignin Participated by Metal (hydro) Oxides. Chin. J. Anal. Chem. 2019, 47, 1809–1815. [Google Scholar]










| Stage | Systems | Cake Filtration | Intermediate Blocking | Standard Blocking | Complete Blocking |
|---|---|---|---|---|---|
| 1 | GDM | 0.960 | 0.972 | 0.961 | 0.983 |
| AS/GDM | 0.912 | 0.923 | 0.938 | 0.938 | |
| AS-CS/GDM | 0.934 | 0.934 | 0.944 | 0.947 | |
| 2 | GDM | 0.892 | 0.953 | 0.974 | 0.992 |
| AS/GDM | 0.843 | 0.931 | 0.972 | 0.954 | |
| AS-CS/GDM | 0.820 | 0.916 | 0.959 | 0.934 | |
| 3 | GDM | 0.859 | 0.952 | 0.998 | 0.983 |
| AS/GDM | 0.855 | 0.942 | 0.976 | 0.963 | |
| AS-CS/GDM | 0.845 | 0.937 | 0.979 | 0.964 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, P.; Li, X.; Yang, Y.; Zhou, Z.; Fan, X.; Feng, J. Algae-Laden Fouling Control by Gravity-Driven Membrane Ultrafiltration with Aluminum Sulfate-Chitosan: The Property of Floc and Cake Layer. Water 2020, 12, 1990. https://doi.org/10.3390/w12071990
Du P, Li X, Yang Y, Zhou Z, Fan X, Feng J. Algae-Laden Fouling Control by Gravity-Driven Membrane Ultrafiltration with Aluminum Sulfate-Chitosan: The Property of Floc and Cake Layer. Water. 2020; 12(7):1990. https://doi.org/10.3390/w12071990
Chicago/Turabian StyleDu, Peng, Xing Li, Yanling Yang, Zhiwei Zhou, Xiaoyan Fan, and Jianyong Feng. 2020. "Algae-Laden Fouling Control by Gravity-Driven Membrane Ultrafiltration with Aluminum Sulfate-Chitosan: The Property of Floc and Cake Layer" Water 12, no. 7: 1990. https://doi.org/10.3390/w12071990