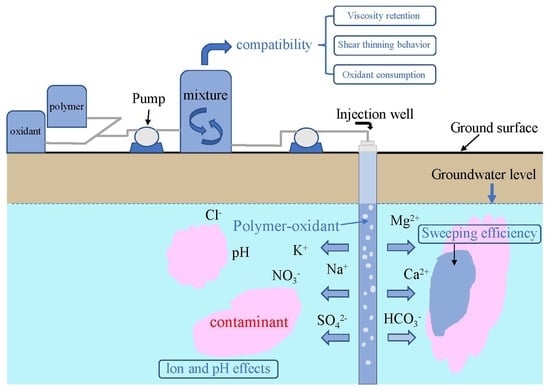
Assessment of the Rheological Behavior of Polymer–Oxidant Mixtures and the Influence of the Groundwater Environment on Their Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Chemical Properties of the Polymer Solutions
3.1.1. Viscosity Profiles for the Polymer Solutions
3.1.2. Viscosity Retention over Time
3.2. Polymer–Oxidant Mixture Compatibility
3.2.1. Viscosity and Rheological Behavior of the Polymer–Oxidant Mixture
3.2.2. Effect of Oxidant Ions on the Solution Viscosity
3.2.3. Oxidant Consumption
3.3. Effects of the Groundwater Environment on the Polymer–Oxidant Mixture
3.3.1. Effect of Common Salt Ions
3.3.2. Effect of pH
3.4. Sweeping Efficiency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Viscosity Profile of Polymers

Appendix B. Viscosity Retention and Shear Thinning Behavior of the Polymer–Oxidant Mixtures


References
- Gates, D.D.; Siegrist, R.L.; Cline, S.R. Chemical Oxidation of Contaminants in Clay or Sandy Soil. In Proceedings of the ASCE National Conference on Environmental Engineering, Technologies for Site Remediation and Hazardous Waste Management, Pittsburgh, PA, USA, 23–26 July 1995. [Google Scholar]
- Lee, B.S.; Kim, J.H.; Lee, K.C.; Kim, Y.B.; Schwartz, F.W.; Lee, E.S.; Woo, N.C.; Lee, M.K. Efficacy of controlled-release KMnO4 (CRP) for controlling dissolved TCE plume in groundwater: A large flow-tank study. Chemosphere 2009, 74, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Schnarr, M.; Truan, C.; Farquhar, G.; Hood, E.; Gonullu, T.; Stickney, B. Laboratory and controlled field experiments using potassium permanganate to remediate trichloroethylene and perchloroethylene DNAPLs in porous media. J. Contam. Hydrol. 1998, 29, 205–224. [Google Scholar] [CrossRef]
- Seol, Y.; Schwartz, F.W. Phase-transfer catalysis applied to the oxidation of nonaqueous phase trichloroethylene by potassium permanganate. J. Contam. Hydrol. 2000, 44, 185–201. [Google Scholar] [CrossRef]
- Yan, Y.E.; Schwartz, F.W. Oxidative degradation and kinetics of chlorinated ethylenes by potassium permanganate. J. Contam. Hydrol. 1999, 37, 343–365. [Google Scholar] [CrossRef]
- Zhang, H.; Schwartz, F.W. Simulating the in situ oxidative treatment of chlorinated ethylenes by potassium permanganate. Water Resour. Res. 2000, 36, 3031–3042. [Google Scholar] [CrossRef] [Green Version]
- Cherry, J.A. Chlorinated solvents in groundwater: Field experimental studies of behaviour and remediation. J. Hazard. Mater. 1992, 32, 275–278. [Google Scholar] [CrossRef]
- Kao, C.M.; Huang, K.D.; Wang, J.Y. Application of potassium permanganate as an oxidant for in situ oxidation of trichloroethylene-contaminated groundwater: A laboratory and kinetics study. J. Hazard. Mater. 2008, 153, 919–927. [Google Scholar] [CrossRef]
- Plumb, R.H., Jr. A comparison of ground water monitoring data from CERCLA and RCRA sites. Ground Water Monit. Remediat. 1987, 7, 94–100. [Google Scholar] [CrossRef]
- Krembs, F.J.; Siegrist, R.L.; Crimi, M.L.; Furrer, R.F.; Petri, B.G. ISCO for Groundwater Remediation: Analysis of Field Applications and Performance. J. Groundw. Monit. Remediat. 2010, 30, 42–53. [Google Scholar] [CrossRef]
- Vella, P.A.; Deshinsky, G.; Boll, J.E.; Munder, J.; Joyce, W.M. Treatment of Low Level Phenols (μg/L) with Potassium Permanganate. Res. J. Water Pollut. Control Fed. 1990, 62, 907–914. [Google Scholar]
- Vella, P.A.; Veronda, B. Oxidation of Trichloroethylene; Comparison of Potassium Permanganate and Fenton’s Reagent. In Proceedings of the Third International Symposium on Chemical Oxidation Technology for the Nineties, Nashville, TN, USA, 17–19 February 1993. [Google Scholar]
- Wiberg, K.B.; Saegebarth, K.A. The Mechanisms of Permanganate Oxidation. IV. Hydroxylation of Olefins and Related Reactions. J. Am. Chem. Soc. 1957, 79, 2822–2824. [Google Scholar] [CrossRef]
- Liang, C.J.; Bruell, C.J.; Marley, M.C.; Sperry, K.L. Persulfate oxidation for in situ remediation of TCE. I. Activated by ferrous ion with and without a persulfate–thiosulfate redox couple. Chemosphere 2004, 55, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.J.; Bruell, C.J.; Marley, M.C.; Sperry, K.L. Persulfate oxidation for in situ remediation of TCE. II. Activated by chelated ferrous ion. Chemosphere 2004, 55, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Kolthoff, I.M.; Miller, I.K. The chemistry of persulfate. I. The kinetics and mechanism of the decomposition of the persulfate ion in aqueous medium. J. Am. Chem. Soc. 1951, 73, 3055–3059. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Waldemer, R.H.; Tratnyek, P.G.; Johnson, R.L.; Nurmi, J.T. Oxidation of Chlorinated Ethenes by Heat-Activated Persulfate: Kinetics and Products. Environ. Sci. Technol. 2007, 41, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Chokejaroenrat, C.; Kananizadeh, N.; Sakulthaew, C.; Comfort, S.; Li, Y. Improving the sweeping efficiency of permanganate into low permeable zones to treat TCE: Experimental results and model development. Environ. Sci. Technol. 2013, 47, 13031–13038. [Google Scholar] [CrossRef] [PubMed]
- Seol, Y.; Zhang, H.; Schwartz, F.W. A review of in-situ chemical oxidation and heterogeneity. Environ. Eng. Geosci. 2003, 9, 37–49. [Google Scholar] [CrossRef]
- Crimi, M.; Silva, J.A.K.; Palaia, T.A. Cooperative Technology Demonstration: Polymer-Enhanced Subsurface Delivery and Distribution of Permanganate; ESTCP Project. No. ER-200912; Environmental Security Technology Certification Program: Alexandria, VA, USA, 2013. [Google Scholar]
- Kananizadeh, N.; Chokejaroenrat, C.; Li, Y.; Comfort, S. Modeling improved ISCO treatment of low permeable zones via viscosity modification: Assessment of system variables. J. Contam. Hydrol. 2015, 173, 25–37. [Google Scholar] [CrossRef]
- Silva, J.A.K.; Smith, M.M.; Munakata-Marr, J.; McCray, J.E. The effect of system variables on in situ sweep-efficiency improvements via viscosity modification. J. Contam. Hydrol. 2012, 136–137, 117–130. [Google Scholar] [CrossRef]
- Comba, S.; Dalmazzo, D.; Santagata, E.; Sethi, R. Rheological characterization of xanthan suspensions of nanoscale iron for injection in porous media. J. Hazard. Mater. 2011, 185, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.I.M.; McCray, J.E.; Currie, P.K.; Zitha, P.L.J. Polymer enhanced DNAPL flushing from low permeability media: An experimental study. Groundw. Monit. Remediat. 2010, 23, 92–101. [Google Scholar] [CrossRef]
- Martel, K.E.; Martel, R.; Lefebvre, R.; Gelinas, P.J. Laboratory Study of Polymer Solutions Used for Mobility Control During In Situ NAPL Recovery. Ground Water Monit. Remediat. 1998, 18, 103–113. [Google Scholar] [CrossRef]
- Martel, R.; Hebert, A.; Lefebvre, R.; Gelinas, P.; Gabriel, U. Displacement and sweep efficiencies in a DNAPL recovery test using micellar and polymer solutions injected in a five-spot pattern. J. Contam. Hydrol. 2004, 75, 1–29. [Google Scholar] [CrossRef] [PubMed]
- McCray, J.E.; Munakata-Marr, J.; Silva, J.A.K.; Davenport, S.; Smith, M.M. Multi-Scale Experiments to Evaluate Mobility Control Methods for Enhancing the Sweep Efficiency of Injected Subsurface; Project. No. ER-1486; Department of Defense Strategic Environmental Research and Development Program (SERDP): Alexandria, VA, USA, 2010. [Google Scholar]
- Robert, T.; Martel, R.; Conrad, S.H.; Lefebvre, R.; Gabriel, U. Visualization of TCE recovery mechanisms using surfactant-polymer solutions in a two-dimensional heterogeneous sand model. J. Contam. Hydrol. 2006, 86, 3–31. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A.K.; Liberatore, M.; McCray, J.E. Characterization of Bulk Fluid and Transport Properties for Simulating Polymer-Improved Aquifer Remediation. J. Environ. Eng. 2013, 139, 149–159. [Google Scholar] [CrossRef]
- Smith, M.M.; Silva, J.A.K.; Munakata-Marr, J.; McCray, J.E. Compatibility of Polymers and Chemical Oxidants for Enhanced Groundwater Remediation. Environ. Sci. Technol. 2008, 42, 9296–9301. [Google Scholar] [CrossRef]
- Truex, M.J.; Vermeul, V.R.; Mendoza, D.P.; Fritz, B.G.; Mackley, R.D.; Oostrom, M.; Wietsma, T.W.; Macbeth, T.W. Injection of Zero Valent Iron into an Unconfined Aquifer Using Shear-Thinning Fluids. Groundw. Monit. Remediat. 2011, 31, 50–58. [Google Scholar] [CrossRef]
- Vecchia, E.D.; Luna, M.; Sethi, R. Transport in Porous Media of Highly Concentrated Iron Micro- and Nanoparticles in the Presence of Xanthan Gum. Environ. Sci. Technol. 2009, 43, 8942–8947. [Google Scholar] [CrossRef]
- Zhong, L.; Oostrom, M.; Wietsma, T.W.; Covert, M.A. Enhanced remedial amendment delivery through fluid viscosity modifications: Experiments and numerical simulations. J. Contam. Hydrol. 2008, 101, 29–41. [Google Scholar] [CrossRef]
- Zhong, L.; Szeesody, J.; Oostrom, M.; Truex, M.; Shen, X.; Li, X. Enhanced remedial amendment delivery to subsurface using shear thinning fluid and aqueous foam. J. Hazard. Mater. 2011, 191, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Oostrom, M.; Truex, M.J.; Vermeul, V.R.; Szecsody, J.E. Rheological behavior of xanthan gum solution related to shear thinning fluid delivery for subsurface remediation. J. Hazard. Mater. 2013, 244, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.E.; Dwarakanath, V.; Meinardus, H.W.; Young, C.M. Mobility control: How injected surfactants and biostimulants may be forced into low-permeability units. Remediation 2003, 13, 59–66. [Google Scholar] [CrossRef]
- Krembs, F.J. Critical Analysis of the Field Scale Application of in situ Chemical Oxidation for the Remediation of Groundwater. Master’s Thesis, Environmental Science & Engineering Division, Colorado School of Mines, Golden, CO, USA, 2008. [Google Scholar]
- Huang, K.C.; Couttenye, R.A.; Hoag, G.E. Kinetics of heat-assisted persulfate oxidation of methyl tert-butyl ether (MTBE). Chemosphere 2002, 49, 413–420. [Google Scholar] [CrossRef]







| Sample | Polymer (mg/L) | Oxidant (mg/L) | Salt (mg/L) | ||
|---|---|---|---|---|---|
| [KMnO4] | [Na2S2O8] b | [KCl] a | [NaCl] a | ||
| Polymer–oxidant | 800, 1600, 3200 | 500 | 1130 | 0 | 0 |
| Polymer–salt | 800, 1600, 3200 | 0 | 0 | 236 | 555 |
| Polymer–water | 800, 1600, 3200 | 0 | 0 | 0 | 0 |
| Pure oxidant | 0 | 500 | 1130 | 0 | 0 |
| Parameters | Value |
|---|---|
| Na+ (mg/L) | 4.98 |
| K+ (mg/L) | 0.49 |
| Mg2+ (mg/L) | 32.70 |
| Ca2+ (mg/L) | 88.23 |
| Cl− (mg/L) | 5.38 |
| NO3− (mg/L) | 10.91 |
| HCO3− (mg/L) | 305 |
| SO42− (mg/L) | 6.47 |
| pH | 8.21 |
| Duration | Pure Polymer | Polymer–Oxidant Mixture | |||||
|---|---|---|---|---|---|---|---|
| (h) | xanthan | HPAM | xanthan–KMnO4 | HPAM–KMnO4 | xanthan–Na2S2O8 | HPAM–Na2S2O8 | |
| STP (%) | 4 | 4.91 | 1.06 | 3.61 | 0.95 | 3.92 | 0.42 |
| 8 | 4.83 | 1.08 | 3.58 | 0.73 | 3.33 | 0.31 | |
| 24 | 4.84 | 1.07 | 3.53 | 0.51 | 2.75 | 0.26 | |
| 48 | 4.61 | 1.07 | 3.49 | 0.45 | 0.45 | 0.11 | |
| 72 | 4.61 | 0.99 | 3.50 | 0.43 | 0.6 | 0.15 | |
| Salt Type | Salt Concentration (10−3 mol/L) | |||||
|---|---|---|---|---|---|---|
| 0.01 | 0.1 | 1 | 10 | 100 | ||
| Apparent viscosity (cP) | NaCl | 5.03 | 5.62 | 5.11 | 4.36 | 4.35 |
| KCl | 5.66 | 4.83 | 4.63 | 4.2 | 4.08 | |
| CaCl2 | 5.32 | 4.87 | 4.71 | 4.2 | 6.38 | |
| MgCl2 | 5.19 | 5.03 | 4.59 | 4.2 | 3.84 | |
| NaNO3 | 5.5 | 5.11 | 5.11 | 4.24 | 4.2 | |
| NaHCO3 | 5.35 | 5.78 | 5.31 | 4.36 | 4.16 | |
| Na2SO4 | 5.11 | 4.95 | 4.83 | 4.67 | 4.47 | |
| Salt Type | Salt Concentration (10−3 mol/L) | |||||
|---|---|---|---|---|---|---|
| 0.01 | 0.1 | 1 | 10 | 100 | ||
| STP (%) | NaCl | 3.13 | 3.07 | 2.68 | 2.74 | 2.52 |
| KCl | 2.70 | 2.91 | 2.91 | 2.31 | 2.35 | |
| CaCl2 | 2.17 | 2.33 | 1.90 | 2.00 | 5.44 | |
| MgCl2 | 2.84 | 2.80 | 2.10 | 2.67 | 2.58 | |
| NaNO3 | 3.53 | 3.42 | 3.26 | 2.82 | 2.35 | |
| NaHCO3 | 3.63 | 4.13 | 3.00 | 2.89 | 1.33 | |
| Na2SO4 | 3.46 | 3.48 | 3.12 | 2.74 | 2.21 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Chen, J.; Song, X. Assessment of the Rheological Behavior of Polymer–Oxidant Mixtures and the Influence of the Groundwater Environment on Their Properties. Water 2019, 11, 1698. https://doi.org/10.3390/w11081698
Xu Q, Chen J, Song X. Assessment of the Rheological Behavior of Polymer–Oxidant Mixtures and the Influence of the Groundwater Environment on Their Properties. Water. 2019; 11(8):1698. https://doi.org/10.3390/w11081698
Chicago/Turabian StyleXu, Qi, Jiajun Chen, and Xinran Song. 2019. "Assessment of the Rheological Behavior of Polymer–Oxidant Mixtures and the Influence of the Groundwater Environment on Their Properties" Water 11, no. 8: 1698. https://doi.org/10.3390/w11081698