In Situ Formation of Ionic Liquid by Metathesis Reaction for the Rapid Removal of Bisphenol A from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Materials
2.2. Instrumentation and Analytical Conditions
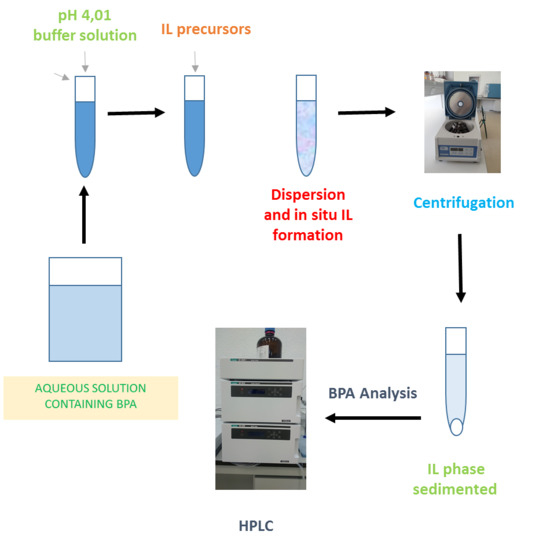
2.3. General Procedure
2.4. Microextraction Kinetics Studies
3. Results and Discussion
3.1. Acceptor Phase—The Ionic Liquid
3.2. Optimization of Removal Conditions
3.2.1. Optimization of Ionic Liquid Precursors Volumes
3.2.2. Optimization of pH Conditions
3.2.3. Optimization of Temperature Conditions and Incubation Time
3.2.4. Analytical Figures of Merit
3.2.5. Recycling and Reuse Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiao, C.M.; Zhuo, J.L.; Chen, X.L.; Li, S.X.; Wang, H.J. Flame retardant epoxy resin based on bisphenol A epoxy resin modified by phosphoric acid. J. Therm. Anal. Calorim. 2013, 114, 253–259. [Google Scholar] [CrossRef]
- Colonna, M.; Berti, C.; Binassi, E.; Fiorini, M.; Sullalti, S.; Acquasanta, F.; Karanam, S.; Brunelle, D.J. Synthesis and characterization of sulfonated telechelic bisphenol A polycarbonate ionomers. React. Funct. Polym. 2011, 71, 1001–1007. [Google Scholar] [CrossRef]
- Hu, L.M.; Zhang, G.S.; Liu, M.; Wang, Q.; Dong, S.Y.; Wang, P. Application of nickel foam-supported Co3O4-Bi2O3 as a heterogeneous catalyst for BPA removal by peroxymonosulfate activation. Sci. Total Environ. 2019, 647, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Lassouane, F.; Ait-Amar, H.; Amrani, S.; Rodriguez-Couto, S. A promising laccase immobilization approach for Bisphenol A removal from aqueous solutions. Bioresour. Technol. 2019, 271, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.D.; Gu, Y.Y.; Lan, H.Z.; Sun, Y.Y.; Gao, H.J. Functional graphene-gold nano-composite fabricated electrochemical biosensor for direct and rapid detection of bisphenol A. Anal. Chim. Acta 2015, 853, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.; Zoltowska-Aksamitowska, S.; Zgola-Grzeskowiak, A.; Ehrlich, H.; Jesionowski, T. Iron(III) phthalocyanine supported on a spongin scaffold as an advanced photocatalyst in a highly efficient removal process of halophenols and bisphenol A. J. Hazard. Mater. 2018, 347, 78–88. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Islam, M.T.; Imam, M.A.; Hyder, A.H.M.G.; Jabbari, V.; Dominguez, N.; Noveron, J.C. Biosorption of bisphenol A and sulfamethoxazole from water using sulfonated coffee waste: Isotherm, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2018, 6, 6602–6611. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Jabbari, V.; Islam, M.T.; Turley, R.S.; Dominguez, N.; Kim, H.; Castro, E.; Hernandez-Viezcas, J.A.; Curry, M.L.; Lopez, J.; et al. Sustainable synthesis and remarkable adsorption capacity of MOF/graphene oxide and MOF/CNT based hybrid nanocomposites for the removal of Bisphenol A from water. Sci. Total Environ. 2019, 673, 306–317. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Yang, L.; Johansson, E.M.J.; Wang, Y.H.; Jin, P.K. Photocatalytic activity and mechanism of bisphenol A removal over TiO2-x/rGO nanocomposite driven by visible light. Chem. Eng. J. 2018, 350, 1043–1055. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I.; Gurmen, S.; Gafarli, I.; Khoei, S.; Safaltin, S.; Ozcelik, D.Y. Oxidative degradation of Bisphenol A by carbocatalytic activation of persulfate and peroxymonosulfate with reduced graphene oxide. J. Hazard. Mater. 2018, 360, 141–149. [Google Scholar] [CrossRef]
- Liu, Z.S.; Wang, H.W.; Ou, J.J.; Chen, L.F.; Ye, M.L. Construction of hierarchically porous monoliths from covalent organic frameworks (COFs) and their application for bisphenol A removal. J. Hazard. Mater. 2018, 355, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Ben Ouada, S.; Ben Ali, R.; Leboulanger, C.; Ben Ouada, H.; Sayadi, S. Effect of Bisphenol A on the extremophilic microalgal strain Picocystis sp (Chlorophyta) and its high BPA removal ability. Ecotox. Environ. Safe. 2018, 158, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kwak, S.Y. Rapid adsorption of bisphenol A from wastewater by beta-cyclodextrin-functionalized mesoporous magnetic clusters. Appl. Surf. Sci. 2019, 467, 178–184. [Google Scholar] [CrossRef]
- Lu, L.; Chen, B.L. Enhanced bisphenol A removal from stormwater in biochar-amended biofilters: Combined with batch sorption and fixed-bed column studies. Environ. Pollut. 2018, 243, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.G.; Vu, H.C.; Le, T.T.; Chang, Y.S. Activation of persulfate by a novel Fe(II)-immobilized chitosan/alginate composite for bisphenol A degradation. Chem. Eng. J. 2018, 353, 736–745. [Google Scholar] [CrossRef]
- Du, H.X.; Piao, M.Y. Facile preparation of microscale hydrogel particles for high efficiency adsorption of bisphenol A from aqueous solution. Environ. Sci. Pollut. Res. 2018, 25, 28562–28571. [Google Scholar] [CrossRef]
- Zahari, A.M.; Shuo, C.W.; Sathishkumar, P.; Yusoff, A.R.M.; Gu, F.L.; Buang, N.A.; Woei-Jye, L.; Gohari, R.J.; Yusop, Z. A reusable electrospun PVDF-PVP-MnO2 nanocomposite membrane for bisphenol A removal from drinking water. J. Environ. Chem. Eng. 2018, 6, 5801–5811. [Google Scholar] [CrossRef]
- Tursi, A.; Chatzisymeon, E.; Chidichimo, F.; Beneduci, A.; Chidichimo, G. Removal of endocrine disrupting chemicals from water: Adsorption of Bisphenol-A by biobased hydrophobic functionalized cellulose. Int. J. Environ. Res. Public Health 2018, 15, 13. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Islam, M.T.; Hernandez, C.; Kim, H.; Lin, Y.; Curry, M.L.; Gardea-Torresdey, J.; Noveron, J.C. Adsorptive removal of sulfamethoxazole and Bisphenol A from contaminated water using functionalized carbonaceous material derived from tea leaves. J. Environ. Chem. Eng. 2018, 6, 4215–4225. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Islam, M.T.; Hernandez, C.; Castro, E.; Katla, S.K.; Kim, H.; Lin, Y.; Curry, M.L.; Gardea-Torresdey, J.; Noveron, J.C. Biomass conversion of saw dust to a functionalized carbonaceous materials for the removal of Tetracycline, Sulfamethoxazole and Bisphenol A from water. J. Environ. Chem. Eng. 2018, 6, 4329–4338. [Google Scholar] [CrossRef]
- Zhu, S.Q.; Wang, L.J.; Su, A.; Zhang, H.X. Dispersive liquid-liquid microextraction of phenolic compounds from vegetable oils using a magnetic ionic liquid. J. Sep. Sci. 2017, 40, 3130–3137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Bai, H.; Xie, G.; Xiao, J. Trace determination of organophosphorus pesticides in environmental samples by temperature-controlled ionic liquid dispersive liquid-phase microextraction. J. Chromatogr. A 2008, 1188, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Mesa, L.B.A.; Padro, J.M.; Reta, M. Analysis of non-polar heterocyclic aromatic amines in beefburguers by using microwave-assisted extraction and dispersive liquid-ionic liquid microextraction. Food Chem. 2013, 141, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, W.; Zhang, T.; Yao, S. Systematic investigation for extraction and separation of polyphenols in tea leaves by magnetic ionic liquids. J. Sci. Food Agric. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wasserscheid, P.; Stark, A. Ionic liquids. In Green Solvent; Wiley: Weinheim, Germany, 2010; Volume 6. [Google Scholar]
- Zhou, Q.X.; Gao, Y.Y.; Xie, G.H. Determination of bisphenol A, 4-n-nonylphenol, and 4-tert-octylphenol by temperature-controlled ionic liquid dispersive liquid-phase microextraction combined with high performance liquid chromatography-fluorescence detector. Talanta 2011, 85, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Fan, Y.C.; Pei, Y.C.; Wu, K.; Wang, J.J.; Fan, M.H. Solvent extraction of selected endocrine-disrupting phenols using ionic liquids. Sep. Purif. Technol. 2008, 61, 324–331. [Google Scholar] [CrossRef]
- Lu, Y.C.; Lin, Q.; Luo, G.S.; Dai, Y.Y. Directly suspended droplet microextraction. Anal. Chim. Acta 2006, 566, 259–264. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhang, D.F.; Xu, X.; Zhang, L. Application of ionic liquid-based dispersive liquid phase microextraction for highly sensitive simultaneous determination of three endocrine disrupting compounds in food packaging. Food Chem. 2016, 197, 754–760. [Google Scholar] [CrossRef]
- Yao, C.; Anderson, J.L. Dispersive liquid-liquid microextraction using an in situ metathesis reaction to form an ionic liquid extraction phase for the preconcentration of aromatic compounds from water. Anal. Bioanalyt. Chem. 2009, 395, 1491–1502. [Google Scholar] [CrossRef]
- Freire, M.G.; Santos, L.; Fernandes, A.M.; Coutinho, J.A.P.; Marrucho, I.M. An overview of the mutual solubilities of water-imidazolium-based ionic liquids systems. Fluid Phase Equilib. 2007, 261, 449–454. [Google Scholar] [CrossRef]
- Baghdadi, M.; Shemirani, F. Cold-induced aggregation microextraction: A novel sample preparation technique based on ionic liquids. Anal. Chim. Acta 2008, 613, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.A.; Dorn, P.B.; Klecka, G.M.; O’Block, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente-Martínez, Y.; Caravaca, M.; Soto-Meca, A.; De Francisco-Ortíz, Ó.; Fernández-López, C. In Situ Formation of Ionic Liquid by Metathesis Reaction for the Rapid Removal of Bisphenol A from Aqueous Solutions. Water 2019, 11, 2087. https://doi.org/10.3390/w11102087
Vicente-Martínez Y, Caravaca M, Soto-Meca A, De Francisco-Ortíz Ó, Fernández-López C. In Situ Formation of Ionic Liquid by Metathesis Reaction for the Rapid Removal of Bisphenol A from Aqueous Solutions. Water. 2019; 11(10):2087. https://doi.org/10.3390/w11102087
Chicago/Turabian StyleVicente-Martínez, Yesica, Manuel Caravaca, Antonio Soto-Meca, Óscar De Francisco-Ortíz, and Carmen Fernández-López. 2019. "In Situ Formation of Ionic Liquid by Metathesis Reaction for the Rapid Removal of Bisphenol A from Aqueous Solutions" Water 11, no. 10: 2087. https://doi.org/10.3390/w11102087