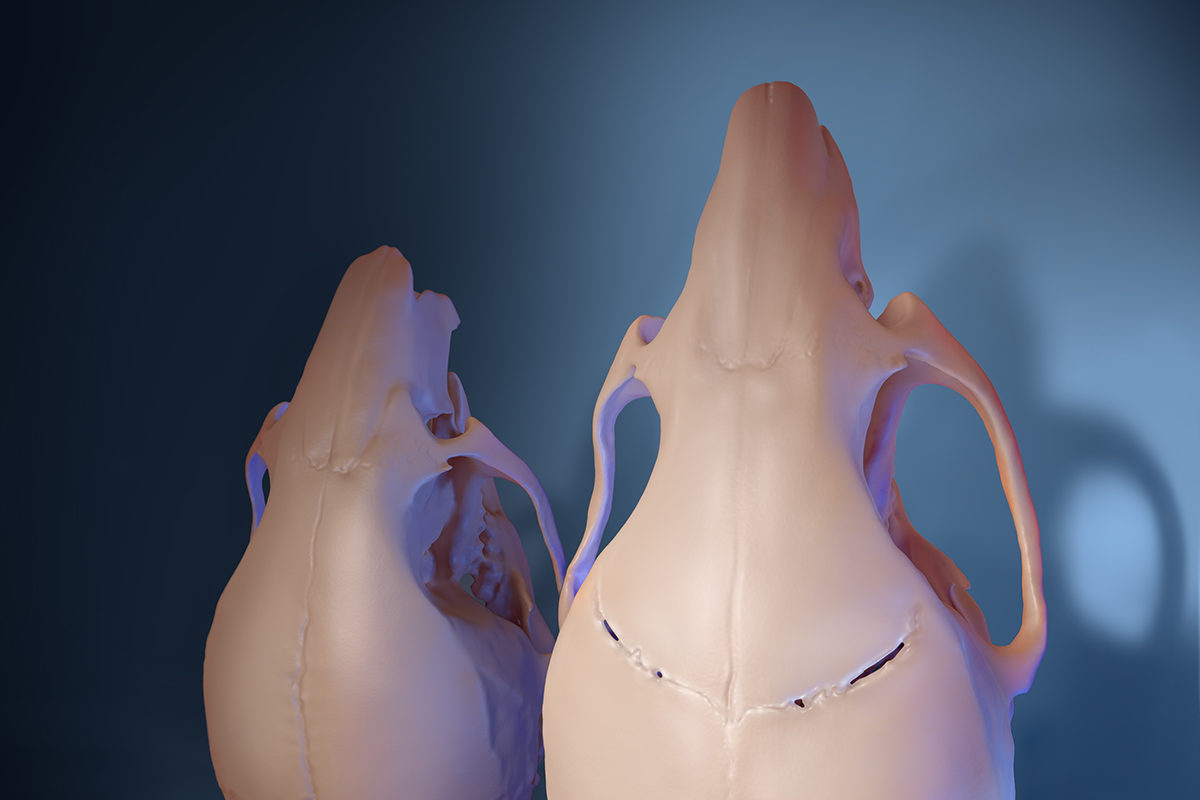
One of our every 2,500 infants born in the United States will suffer from craniosynostosis — a craniofacial defect caused by the premature fusion of the different bones that comprise the human skull.
In a typically developing infant, these bones are separated by fibrous joints (think: “soft spots”) that allow for the skull’s continuing expansion as the brain grows.
When an infant develops craniosynostosis, the brain continues to grow while the skull does not, causing anything from a misshapen skull (in mild cases) to development delays, hearing loss, blindness and even death (in more severe cases).
Until now, the standard treatment has been for craniofacial surgeons to break apart the skull, using plates and screws to separate the bones and hold them in place. It’s an incredibly invasive surgery that often has to be done more than once.
Recreating and Treating Craniosynostosis in Mice
But this treatment could become a thing of the past, according to an article titled, “Cranial suture regeneration mitigates skull and neurocognitive defects in craniosynostosis,” published in the journal Cell online recently.
In the NIDCR-funded study, USC researchers were able to show that mice with a mutation in the TWIST1 gene also suffer from neurological and cognitive aspects of craniosynostosis, just like in humans. But no animal models have previously been established for some of the more devastating neurocognitive symptoms beyond those affecting the bones themselves.
Building on knowledge they had previously discovered, including the role of the Gli1+ stem cells in keeping sutures from prematurely fusing, the group added the Gli1+ stem cells to a biodegradable gel and placed the mixture in the sutures.
Six months later, new fibrous sutures had formed in the treated areas, according to skull imaging and tissue analysis.
The discovery is the closest we’ve come to treating craniosynostosis without overly invasive (and often multiple) surgeries.
“I started my career as a clinician treating kids with congenital defects, and we always wanted to do something better for these patients,” said Yang Chai PhD ’91, DDS ’96, University Professor, Ostrow’s Associate Dean of Research and Director of the Center for Craniofacial Molecular Biology. “I think, with this stem cell-mediated suture regeneration approach, it truly gives us the hope that one day we can apply this as a biological solution for this biological problem.” Chai is also the lead of this study.
“As someone who operates weekly on babies with craniosynostosis, this work could someday put me out of a job,” said Mark Urata, Chief of the Division of Plastic Surgery and Reconstructive Surgery at Keck School of Medicine of USC and study contributor. “Currently, these are operations that involve moving the bones of the forehead and skull while operating on top of the brain. To be able to avoid those risks would obviously be a tremendous paradigm shift for the field of craniofacial surgery.”
In addition to his duties at Keck, Urata is also the Chair of the Division of Oral and Maxillofacial Surgery at Ostrow and the Head of the Division of Plastic and Maxillofacial Surgery at the Children’s Hospital of Los Angeles.
More Than Aesthetics
The discovery could lead to much more than simply fixing an aesthetic condition; it could be life-altering for those experiencing neurocognitive issues resulting from craniosynostosis. Teaming up with Associate Professor Jian-Fu Chen and his research team, who focus on neural development and neurodegenerative disease, the interdisciplinary group began to look at the link between craniosynostosis and the elevated intracranial pressure that arises from craniosynostosis, which can lead to learning deficiencies and neurocognitive behavioral issues.
“The connection between changes in the skull and the development of cognitive deficits had not been fully explored,” Chai said. “We wanted to know if restoring sutures could improve neurocognitive function in the mice.”
Before the intervention, the mice with craniosynostosis had increased pressure inside their skulls and performed poorly on social and spatial memory and motor learning tests. After treatment, these measures all returned to levels typical of healthy mice.
“We have always suspected that craniosynostosis might create pressure on the brain and that, in turn, could create developmental disadvantages for these children,” Urata said. “Side by side with our work to regenerate a functioning suture, this could be life changing for so many babies yet to be born.”
“I hope we will be able to use this approach to regenerate a biological suture, and then the infants’ skulls and brains will develop just like normal,” Chai added. “They will basically be starting at the same point as everyone else instead of having any type of neurocognitive abnormalities that require extra help in their studies. So they will be able to play as children or advance in their careers as adults on equal footing.”
Next up for the study will be to scale up to larger animals before entering a Phase 1 clinical trial on human beings.
A Truly Collaborative Effort
This high-impact discovery represents a truly collaborative group of clinicians and researchers from all around the world. In addition to Chai and Urata, Feng and his postdoctoral fellow Li Ma performed all the neurocognitive studies.
Other authors of the study include the Center for Craniofacial Molecular Biology’s Mengfei Yu (study lead author), Yuan Yuan, Jinzhi He and Thach-Vu Ho; and the Zilkha Neurogenetic Institute’s Axel Montagne, Yingxi Wu, Zhen Zhao, Naoma Sta Maria, Russell Jacobs and Berislav V. Zlokovic participated. Additionally Xin Ye and Huiming Wang, of the Key Laboratory of Oral Biomedical Research at the Zhejiang University School of Medicine in Hangzhou in China, were involved.
“It was just really beyond what I could ever have asked for,” Chai said of the group’s effort. “It was really was just like all the stars aligned. I have no words to describe how grateful I am for all the collaboration.”
Added Urata, “Forging a bond between a world-class craniofacial research center (the Center for Craniofacial Molecular Biology) and a world-class craniofacial care center (Children’s Hospital of Los Angeles) cross schools (medicine and dentistry) and disciplines for the sake of improving care for children. This is one of the truly special aspects of USC.”
This research was supported by NIDCR grants DE026339, DE012711, DE022503, and DE026914. Support also came from the National Institute of Neurological Disorders and Strokes grant NS097231.
