Abstract
A pipe rack assembly uses hot-dip galvanized trusses joined together by screw connections. The welded trusses were manufactured with ASTM A572 gr.50 W steel profiles. Two of them fractured during the galvanizing bath. The fractures presented typical characteristics of the LMAC — liquid metal-assisted cracking mechanism. In both cases, the fracture was located in the structural element base metal and followed the fillet weld to the neighboring elements at a distance beyond the heat-affected zone. The trusses height was greater than the depth of the molten zinc vat, and double immersion is required for complete galvanization. The failed trusses had a particular geometry, distinct from the others. The design of the trusses was verified taking into account the thermal stresses generated by the partial immersion in the zinc bath. Among all, only the two broken trusses were exposed to stresses of thermal origin substantially higher than the material yield strength. Metallographic analysis revealed secondary intergranular cracks near the fracture surface. The crack roots contained zinc. In addition, the secondary cracks had a large opening in relation to the depth, which indicates material plasticization in the root region.
Similar content being viewed by others
Introduction
The pipe rack, object of this paper, was assembled by the screw connection of 226 trusses. Some have a specific arrangement, others have common geometric characteristics in such a way that it is possible to classify the set of trusses in 23 different arrangements. All were manufactured by welded union of W profiles produced by controlled rolling of ASTM A572 gr.50 steel [1], which is a low-alloy, high-strength steel often used in the construction of welded, bolted or riveted metal structures. The material’s quality certificates and welding procedures followed good engineering practices and met project specifications. After manufacture, the trusses were hot-dip galvanized. Due to its dimensions, galvanizing was conducted by double immersion in the zinc bath, as shown in Fig. 1. According to Mannheimer and Cabral [2], this is a classic solution in the case of large parts.
During the pipe rack assembly, visual inspection identified two trusses containing a fractured structural element. In both cases, the through crack was in the vicinity of one of the welded connections of the element to the rest of the truss. The crack started at one of the flanges and propagates in part of the web extension, without reaching the opposite flange. The fracture was located in the base metal of the element and followed the weld to a connection thread outside the heat-affected zone. The two damaged trusses had different arrangements due to the dimensions of the respective structural elements and did not resemble any other trusses of the pipe rack. Figure 2 shows the fracture location in one of these trusses.
This failure analysis was developed to answer three questions: (1) what the failure mechanism is and how it works; (2) what to expect from the structural integrity of the other trusses of the pipe rack; (3) what to do to prevent a recurrence of failure in the future hot-dip galvanizing of large trusses.
Bibliographic Review
Hot-dip galvanizing as a means of protecting steel against corrosion has been used since the 19th century and the first specification published by ASTM on galvanized coating dates from 1928 [3]. Under a thermodynamic aspect, zinc offers low resistance to corrosion. However, zinc in contact with approximately neutral solutions tends to form a layer of corrosion products that restrict the access of hydrogen, oxygen and OH ions to the metal surface. This passive property gives to zinc an outstanding resistance to atmospheric corrosion, counteracting the thermodynamic trend.
As long as the zinc coating applied to a truss remains continuous, the structure behaves in respect to corrosion as if it was made of zinc. An electrochemical characteristic of zinc manifests itself when there is a localized loss of continuity of the coating, whether resulting from mechanical impact, or even due to its own corrosion. In this case, the truss area exposed to contact with the atmosphere remains protected due to the dissolution of the surrounding zinc layer.
The service life of the galvanized protection of a truss depends on the characteristics of the environment to which it is exposed, the initial thickness of the zinc layer and its continuity. The minimum thickness of the zinc layer is specified. For laminated steel profiles with a wall thickness equal to or greater than 6mm, the ABNT NBR 6323 [4, 5] specification establishes a minimum zinc layer thickness of 74 µm in an individual sample and 84 µm in the average between representative samples.
Generally speaking, the hot-dip galvanizing process is subdivided into the following steps [3, 6]: (1) degreasing; (2) pickling; (3) fluxing; (4) drying; and (5) zinc bath.
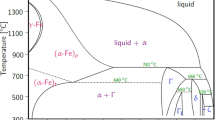
Liquid zinc reacts with iron and other elements present in steel. Under normal galvanizing conditions, Fe diffuses into Zn to form a series of layers consisting of intermetallics from the Zn-Fe system, as shown in Fig. 3 [2, 7]. Thus, the following layers have a decreasing iron content in the order they are deposited on the steel surface [6].
-
(a)
The gamma1 (г1) phase, Fe5Zn21, with an iron composition of 17-19.5 wt.%. The thickness is about 1µm.
-
(b)
The delta (δ) phase, FeZn10, has an iron composition range of 7.0-11.5 wt.%.
-
(c)
The zeta phase (ξ), FeZn13, has an iron content of approximately 5-6 wt.%.
-
(d)
Layer η constituted by the zinc dragged when the truss is removed from the bath. The zinc content in this layer is high.
Zn-Fe system intermetallic layers resulting from the hot galvanizing of a carbon steel: A) typical galvanizing microstructure (1) gamma prime phase (г1); (2) delta phase (б); (3) zeta phase (ζ) B) zinc-rich corner of the Fe-Zn binary phase diagram [6]
The г1 layer thickness changes little, while in the other layers, the thickness depends on galvanizing process variables, as: (1) zinc bath temperature; (2) zinc bath composition; (3) surface roughness resulting from the pickling and fluxing steps; (4) steel composition; (5) entry and exit speed of the truss from the molten zinc vat; and (6) contact duration between the truss and the molten zinc. With the exception of the η layer, with a high Zn content, the others have high hardness and low ductility. In galvanized parts intended for later forming, which does not apply to trusses, it is desirable to inhibit the presence of intermetallic phases.
The pure zinc melting point is 419.5 °C. The galvanizing process standard temperature is in the range of 440 to 470 °C. The higher temperature increases the fluidity of the liquid zinc, benefiting its drainage when the truss is removed from the bath. In contrast, it accelerates the formation of oxides on the bath surface, increases zinc solidification time with the possibility of intensifying the formation of intermetallic layers and favors the warping of the truss due to thermal stresses. Above 480 °C, the dissolution rate of iron in zinc is very high, which affects the performance of the bath, as well as the walls of the vat.
There is no consensus in the literature of the effect of the residual elements present in the zinc bath on the galvanized product. Furthermore, there is a trend in Brazil and abroad for the galvanizers to treat the composition of the bath as an industrial secret. Table 1 outlines the influence attributed to the elements commonly found in zinc baths [2, 8]. Other variables that interfere with the characteristics of the hot-dip galvanizing layer are [2, 6]:
-
a)
Low carbon, silicon and manganese content in the composition of the steel favor smaller thicknesses of the intermetallic layers. Silicon is considered to have the greatest influence among the three elements.
-
b)
The surface roughness contributes to the increase in the intermetallic layers thickness since it increases zinc and steel contact area. The roughness can be controlled through the pickling and fluxing operations.
The occurrence of LMAC — liquid metal assisted cracking, during the hot-dip galvanizing process has been recognized since the 1930s, and the influence of external loading on this type of damage has been documented since the 1980s [9,10,11]. Despite the effort applied in research in the last 30 years, there is still no definitive understanding about the nature of nucleation and crack propagation due to the LMAC effect [12, 13].
The diversity and amplitude of recent work on the study of LMAC during hot-dip galvanizing suggest that this mechanism has become a relevant industrial concern, especially in Europe, in the last few years. During that period, some galvanizers, which until then used lead-containing zinc alloys in their baths, replaced this element with tin and bismuth for economic reasons and ecological pressure. Apparently, this change in the process favored LMAC activity [8, 11, 14].
Despite the lack of understanding about the fundamentals of LMAC during hot galvanizing, there is a consensus in the literature that cracks occur, while steel maintains contact with the zinc bath. The characteristic aspect of the cracks resulting from LMAC allows identifying its origin. The fracture is classified as brittle, since it occurs in the absence of plastic strain on a macroscopic scale, despite the steel's ductile behavior in tensile test and satisfactory tenacity after galvanizing. The propagation is mainly intergranular, and the space between the crack faces is filled by chemical elements contained in the galvanizing bath.
According to Kinstler [3], the activity of the LMAC mechanism in hot-dip galvanizing requires the combination of the following factors: (1) susceptibility of the steel; (2) composition of the bath; and (3) mechanical loading. In general, no single factor promotes the LMAC fracture, but if one of them assumes a particularly aggressive condition, LMAC failure can occur even if the other factors maintain relative smoothness.
The fact that LMAC activity is dictated by the intersection between sets of variables not exactly defined makes it difficult to predict the mechanism's behavior. Therefore, the preventive measures are of a qualitative nature and faced casuistically to the moderation of each individual variable.
Results
The bath composition used to galvanize the trusses in % weight was: 0.0088 Al; 0.648 Pb; 0.0075 Sn; Bi not detected; 0.0376 Fe; 0.0224 Ni; 0.0177 Si; Cd not detected; bal Zn.
Material extracted from the sample highlighted in Fig. 2 from one of the fractured trusses was subjected to chemical analysis with the following results in % weight: 0.158 C; 0.91 Mn; 0.28 Si; 0.036 P; 0.026 S; < 0.003 Nb; 0.014 V; 0.02 Al; 0.08 Cr; 0.04 Ni; 0.04 Mo; 0.317 Cu; and 0.014 Co.
The results of the mechanical tests carried out at room temperature are: 384 MPa yield limit; 533 MPa resistance limit; 22% elongation; 57% area reduction and 85 HRB hardness. The average energy absorbed at 23 °C in Charpy V impact tests applied to 10 × 5 × 55 mm specimens extracted from the flange was 54 J, which represents 108 J in standard specimens.
Metallographic samples containing the fracture surface were extracted from the structural element flange and web and observed by optical microscopy and scanning electron microscopy. The preparation of the metallographic surfaces was obtained by grinding with 100 to 1200 waterpaper, followed by polishing with 1µm diamond paste and finishing with 1µm alumina paste. Most micrographs were obtained after etching by the reagent Nital 2%, as is usual in the case of carbon steels, the rest being produced by the reagent Picral. The zinc layer protects the carbon steel substrate from 2% Nital etching resulting in an unetched region as shown in Fig. 4. The general microstructure of the material documented in Fig. 5 is consistent with that of a high-strength carbon steel with a maximum carbon content of 0.23% by weight, produced by controlled rolling and intended for structural application. It is noteworthy that the microstructure varies little along the wall thickness of the flanges and the profile web. Non-metallic inclusions are of little relevance in size, quantity, distribution and shape. The fracture characteristics are documented in Figs. 6, 7, 8.
Fracture in the flange region. (a) Highlight aspects: (1) The fracture surface contains remnants of zinc; (2) detail K indicates a large secondary crack containing zinc; (3) details L and M show microcracks, all of which have a large opening in relation to depth. (b) The lamination bands keep the shape up to the edge of the fracture surface, indicating that it occurred without the material incorporating significant plasticization. The fracture surface contains zinc deposit. Mechanical polishing. Longitudinal section
Microcrack in the flange region and close to the fracture surface. Propagation is intergranular with branching. The opening is large in relation to the depth. Intermetallics are present at the root of the microcrack which denotes a high capacity for infiltration of the liquid through capillarity inside small openings. Mechanical polishing. Attack by Picral. In B and C images by scanning electron microscopy, in D semi-quantitative chemical analysis by EDS
Fracture surface in the vicinity of the welded fillet joint applied to the web of the broken structural element. It is noteworthy that the fracture surface and the secondary through crack are outside the heat-affected zone and outside the region subjected to the main residual welding stress. Mechanical polishing. Etching: Nital 2%
Discussion
The morphology of the fracture and associated secondary cracks are typical of the LMAC mechanism. It is noteworthy: (1) the brittle nature of the fracture, Fig. 6, although the broken structural element presents ductile behavior in tensile after galvanizing, characterized by an area reduction in tensile test of 57%; (2) secondary cracks and microcracks present in the vicinity of the fracture, all with a large opening in relation to the depth, Figs. 6a and 7a; and (3) the presence of zinc on the fracture surface, as well as on the root of the secondary cracks, Figs. 6 and 7. Note that these features are not consistent with defects resulting from welding or any other stage of manufacturing and of galvanizing prior to their contact with molten zinc.
Other details of interest pointed out by the metallographic analysis are:
-
(a)
The main crack was nucleated and has propagated through the flange of the structural elements exposed to the tensile overload promoted by the partial immersion of the truss in the molten zinc vat.
-
(b)
The fracture is located between 6 to 8 mm from the fillet-welded joint applied to the flange and the web of the structural element, which places it outside the weld heat-affected zone.
-
(c)
Secondary cracks and microcracks are essentially simple, generally of intergranular propagation and ramifications.
-
(d)
The metallographic analysis did not identify the presence of secondary cracks, or microcracks, beyond the immediate vicinity of the fracture.
In order to identify the cause of the fracture, it is necessary to check the aggressiveness of the determining factors for the activity of the LMAC mechanism [3].
Susceptibility of the Material
Chemical analysis and mechanical tests show that the material of the fractured structural element meets the requirements of the ASTM A572 gr.50 specification. The general microstructure of the steel does not have any characteristic that may stimulate fracture. Note that this steel is frequently used in the construction of hot-dip galvanized structures in Brazil, with no record of inadequacy for use. In addition, the ASTM A572 gr.50 specification is equivalent to the European Community specification S355JR whose resistance to the LMAC mechanism during hot-dip galvanizing is considered high [8]. Thus, it appears that the material's susceptibility factor does not represent a significant stimulus for the performance of LMAC in the present case.
Bath Composition
The zinc bath used in the trusses galvanizing does not deviate from the current industrial practices recorded in Table 1. In the vicinity of the fracture, the deposited layer consisting of intermetallic compounds of the Zn Fe system and metallic zinc, Fig. 4, is compatible with the literature records, Fig. 3. Its nominal thickness is 0.18mm. The literature relates the increase in the frequency of failure during hot-dip galvanizing in Europe in the last ten years with the use of a zinc bath containing additions of tin and bismuth [13]. As a preliminary matter, levels of tin and bismuth above 0.15% by weight are not recommended [15]. The bath used does not have a significant presence of elements that favor the occurrence of LMAC. In addition, the zinc content meets the minimum limit of 98% established in the ABNT NBR 6323: 2007 standard [4, 5]. Since the composition of the bath and the layer deposited in the galvanizing meets the industry's good practice, it is considered that the contribution of this susceptibility factor to the activity of the LMAC mechanism is insignificant.
Mechanical Loading
Once the truss is partially submerged in the zinc bath, the load applied to the connection between its structural elements results mainly from a combination of: (1) residual welding stresses; (2) residual lamination stresses; (3) local thermal stresses resulting from the rise in temperature along the wall thickness; and (4) stresses resulting from the restriction offered by coldest part to the thermal expansion of the complementary part of the truss in contact with the molten zinc.
The partial immersion of the trusses in the molten zinc bath had not been considered in the initial design of the trusses. The project was then checked for such condition. This review initially considers the elastic behavior of the material. In the second instance, it adopts viscoelastic behavior for those trusses that, according to the initial stage, have a structural element exposed to substantial plastic strain. The temperature established for the material in the immersed segment is 445 °C, and in the dry segment, it is 70 °C. The flow limit of 397 MPa arbitrated for the material at room temperature corresponds to the minimum flow recorded in the quality certificates of the profiles used in the manufacture of the trusses. At 445 °C, the flow limit of 357.3 MPa was adopted, which is equivalent to 90% of this property at room temperature.
By elastic criteria, the yield limit of the material would have been largely exceeded during the partial immersion in the zinc bath only in the specific structural element broken by LMAC. The maximum tensile stress calculated by elastic criterion in all other elements of these two trusses remains below 60.5% of the material yield limit. The viscoelastic analysis shows that the stress redistribution caused by localized plasticization has little effect on the tensile stresses acting on the other structural elements. The elastic criterion tends to overestimate the tension effectively applied in case of plasticization and to underestimate the stresses in the others, while the opposite is true for the viscoelastic criterion. The set of numerical results confirms that the only structural elements intensely plasticized by thermal overload during partial immersion in the zinc bath in the two trusses were those that had fractured. Bearing in mind that in the present case, the maximum tensile stress applied to the connection between the broken structural element and the rest of the truss results mainly from a flexing load, and the most intense plasticization coincides with the flange where the fracture started.
The three structural elements exposed to the highest tensile stress due to the double immersion were identified by elastic criteria design verification of all trusses of the pipe rack. The stresses were 107.3%, 77.0% and 71.7% of the yield limit. These three structural elements were free of cracks resulting from LMAC. The inspection was carried out by magnetic particles using a procedure specifically qualified for this purpose.
226 trusses were galvanized. Assuming the conservative average of 15 structural elements per truss, there are about 2.700 structural elements. In this universe, only two structural elements fractured by LMAC, precisely those that stood out due to intense plasticization inside the zinc bath. The composition of the bath and the susceptibility of the material, in addition to not have shown importance in the present case, are factors that similarly affect the complete set of trusses, without the discretionary character presented by the plasticization that points exactly to where the fractures occurred. In view of this circumstance, the thermal overload applied to the trusses during double-dip galvanizing is identified as the root cause of fracture. The geometry of the fractured trusses proved to be inadequate for double-dip galvanizing. As a corollary, it is possible to affirm that the galvanizing by double immersion of a truss is safe, as long as the geometry is favorable to this procedure, which is an indispensable design consideration.
The analysis of the factors that contribute to the activity of LMAC shows that the intense plasticization of the material inside the bath was decisive for the fracture. The link between plasticization in the bath and the activity of the LMAC mechanism has been recorded in the literature [9]. The metallographic examination shows in Figs. 5a and 6 the large opening of the secondary cracks and microcracks in relation to the respective depths. In the absence of marked loss of mass due to corrosion, this characteristic of discontinuities is only possible in the event of intense plasticization of the material in the region located ahead of the root. There is thus an apparent contradiction, that is, although the final fracture resulting from LMAC is of a fragile nature, the performance of this mechanism demands intense plasticization of the material.
Before being a contradiction, the combination between plasticization and fragility supports the following model of crack propagation by LMAC during hot galvanizing:
-
a)
The liquid zinc in contact with the steel at the root of a crack quickly attacks the grain boundaries to form with iron a protective film of a low-ductile ZnFe intermetallic.
-
b)
Immediately after formation, this film breaks when it does not follow the strain imposed on the base metal by the localized mechanical stress, whatever its nature.
-
c)
Liquid zinc comes back into contact with steel, renewing the attack and the process is repeated.
According to industry practice, the immersion time of a structure in the galvanizing vat is relatively short, about a few minutes. So for a LMAC crack to manifest, it must be nuclear and propagate quickly. For this, it depends on the activity of the intermetallic film formation reaction and the strain rate imposed on the structure. If one of these contributions progresses slowly, LMAC does not manifest. Note that the formation of intermetallic incorporates the effects of the susceptibility of the material and the composition of the bath to the proposed model. The model also discriminates the nature of the required plastic strain. Crack propagation by LMAC requires a sustained strain, and it is still reasonable to expect it to exceed a certain threshold.
This same model can explain the initial stage of nucleation of cracks by LMAC from sites where pre-existing imperfections favor the concentration of plastic strain. The multiple nucleation of cracks by LMAC observed in Fig. 6a reinforces this possibility.
The crack propagation by LMAC during the galvanizing process depends directly on the localized strain presented by the truss. It justifies the reformulation of the classic diagram that explains the contributing factors for this kind of damage. It is proposed to replace “mechanical loading” usually considered a factor on the literature by “strain”, Fig. 9.
Conclusions
The activity of LMAC — liquid metal assisted cracking, in the galvanizing process requires that the material be subjected to a certain strain while remaining in contact with the zinc bath. The factors that by combination of effects, control this damage mechanism during the galvanizing process are: material susceptibility, bath composition and material strain.
The fracture of two of the 226 trusses used in the assembly of a pipe rack during galvanizing has resulted of LMAC damage. The immediate cause was the intense mechanical overload of thermal origin applied to these trusses inside the zinc bath. The root cause lies in the mechanical design that did not take into account the galvanizing of the trusses by double immersion.
In the conditions equivalence of the material susceptibility and bath composition, the evidence indicates that no other truss, apart from the two that fractured, has a structural element exposed to the strain inside the bath sufficient to promote LMAC activity. The galvanizing of complex structures by double immersion in the molten zinc vat is safe as long as the design verification ensures that the thermal stresses introduced by this procedure do not exceed the material yield strength limit.
References
“Standard Specification for High-Strength Low-Alloy Colummbium-Vanadium Structural Steel” ASTM A572-07: Annual Book of ASTM Standards 2006, section 1, vol. 0104, p 309-312.
W.A. Mannheimer, E.R. Cabral, Galvanização (Ao Livro Técnico, Rio de Janeiro, 1979), p. 100–101. (in Portuguese)
T.J. Kinstler, Current Knowledge of the Cracking During Galvanizing (GalvaScience LLC, Canada, 2005), p. 2
“Galvanização por Imersão a Quente de de Produtos de Aço ou Ferro Fundido – Especificação”, NBR 6323:2016, Associação Brasileira de Normas Técnicas, ABNT, parte 4.1.1.
“Standard Specification for Zinc (Hot-Dip Galvanized) Coatings on Iron and Steel Products”, ASTM A123/A123M − 17, American Society for Testing and Materials, A05.13
Hot Dip Galvanized Coatings; in: Metals Handbook: Surface Cleaning, Finishing, and Coating; ASM International; V.5, 1982, United States; p. 323–332.
A.R. Marder, The metallurgy of zinc coated steel . Prog. Mater. Sci. 45, 191–2715 (2000)
W. Rudd et al., Failure Mechanisms During Galvanizing. European Commission, Directorate-General for Research; EUR 23195 EN; p. 5–20, 52; 2008.
M. Feldmann, Hot-Dip-Zinc-Coating of Prefabricated Structural Steel Components. JRC Scientific and Technical Reports; European Commission; EUR 24286 EN; p. 13, 14; 2010.
M. Neil James, Designing against LMAC in galvanized steel structures. Eng. Fail. Anal. 16, 1051–1061 (2009)
M. Sun et al., Hot-Dip Galvanizing of Cold-Formed Steel Hollow Sections: A State-of-The-Art Review. Struct. Civ. Eng. 13(1), 49–65 (2019)
K.D. Bauer et al., A First Principles Investigation of Zinc Induced Embrittlement at Grain Boundaries in BCC Iron. Acta Mater. 90, 69–76 (2015)
J. Carpio et al., Environmental Factors in Failure During Structural Steel Hot-Dip Galvanizing. Eng. Fail. Anal. 16, 586 (2009)
C. Leighfield et al., The conclusion from one million tons of experience in galvanizing steel – LMAC is not a primary instigator of cracking. Eng. Fail. Anal. 106, 104151 (2019)
E.E. Glickman, Grain Boundary Grooving Accelerated by Local Plasticity as a Possible Mechanism of Liquid Metal Embrittlement. Interface Sci. 11, 451–459 (2003)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silveira, T.L., Perez, I.U., da Silveira, T.F. et al. Liquid Metal-Assisted Cracking During Hot-Dip Galvanizing of Complex Structures: A Case Study. J Fail. Anal. and Preven. 21, 1652–1661 (2021). https://doi.org/10.1007/s11668-021-01211-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11668-021-01211-w