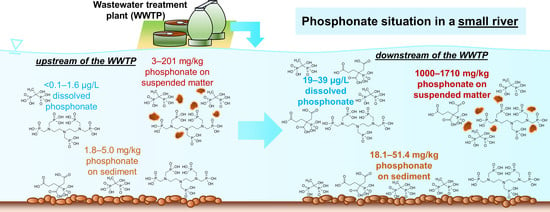
Influence of Wastewater Discharge on the Occurrence of PBTC, HEDP, and Aminophosphonates in Sediment, Suspended Matter, and the Aqueous Phase of Rivers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Concept
2.1.1. General Information
2.1.2. River1 and WWTP1
2.1.3. River2 and WWTP2
2.1.4. River3 Sediment
2.2. Sampling Procedure
2.2.1. Liquid Random Samples
2.2.2. Sediment
2.2.3. Suspended Matter
2.3. Analytical Methods
2.3.1. Flow Rates
2.3.2. Suspended Matter Concentration
2.3.3. Sample Preparation for Phosphonate Analysis
2.3.4. Phosphonate Analysis per IC-ESI-MS/MS
2.4. Statistical Evaluation
3. Results and Discussion
3.1. Dissolved Phosphonates (Liquid Random Samples)
3.1.1. Impact of WWTP
3.1.2. Possible Environmental Impacts
3.2. Phosphonates Adsorbed to Sediment
3.2.1. Individual Analysis of Different Sediment Samples
3.2.2. Impact of WWTP
3.2.3. Phosphonate Distribution in Different Layers of Sediment
3.2.4. Comparison with Literature
3.3. Phosphonates Adsorbed to Suspended Matter
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Sample | GPS Coordinates | |
|---|---|---|
| WWTP1/River1 (Neckar) | ||
| Random & suspended matter sample (before WWTP discharge) | 48.838389° | 9.217667° |
| Staff gauge | 48.838972° | 9.225972° |
| Sediment samples A1 & A2 | 48.839042° | 9.226697° |
| Sediment samples A3 & A4 | 48.838944° | 9.225989° |
| Sediment samples A5 & A6 | 48.840700° | 9.231569° |
| Sediment samples A7 & A8 | 48.842014° | 9.235975° |
| Sediment samples A9 & A10 | 48.842556° | 9.237314° |
| Sediment sample A11 | 48.838453° | 9.226544° |
| Sediment sample A12 | 48.838464° | 9.227325° |
| WWTP discharge point | 48.848306° | 9.245583° |
| Random & suspended matter sample (behind WWTP discharge) | 48.861750° | 9.255333° |
| Sediment sample B1 (close to WWTP) | 48.855917° | 9.251108° |
| Sediment samples B2 & B3 | 48.863006° | 9.255792° |
| Sediment sample B4 | 48.861456° | 9.254706° |
| Sediment samples B5 & B6 | 48.861411° | 9.254250° |
| Sediment samples B7 & B8 | 48.860744° | 9.253806° |
| Sediment samples B9 & B10 | 48.863692° | 9.256000° |
| Sediment sample B11 | 48.863050° | 9.255214° |
| Sediment sample B12 | 48.862575° | 9.254783° |
| Sediment sample B13 | 48.862250° | 9.254631° |
| WWTP2/River2 (Körsch) | ||
| Random sample & sediment samples (before WWTP discharge) | 48.716768° | 9.163746° |
| Suspended matter sample (before WWTP discharge) | 48.716624° | 9.167035° |
| WWTP discharge point | 48.716298° | 9.169461° |
| Random sample, sediment samples, and suspended matter sample (behind WWTP discharge) | 48.713647° | 9.175183° |
| River3 (Rhine) | ||
| Sediment sample for depth profile | 48.101389° | 7.580611° |





| Figure | River | Concentration | Mean (Before WWTP) | Mean (Behind WWTP) | Var. (Before WWTP) | Var. (Behind WWTP) | t | p |
|---|---|---|---|---|---|---|---|---|
| 2 Top-left | 1 | µg/L diss. | 4.25 | 4.30 | 1.24 | 1.96 | −0.0815 | 0.9360 |
| 2 Top-right | 2 | µg/L diss. | 0.60 | 25.88 | 0.36 | 45.16 | −11.851 | <0.0001 |
| 2 Bottom-left | 1 | mg/kg sed. | 17.30 | 32.37 | 51.60 | 135.79 | −3.4814 | 0.0033 |
| 2 Bottom-right | 2 | mg/kg sed. | 3.31 | 30.53 | 1.02 | 120.95 | −7.7938 | <0.0001 |
| 8A | 1 | mg/kg SM | 81.11 | 75.83 | 8535.06 | 4703.47 | 0.0795 | 0.9405 |
| 8C | 1 | µg/L SM | 0.41 | 0.60 | 0.03 | 0.10 | −0.9562 | 0.4095 |
| 8E | 1 | µg/L SM & diss. | 4.26 | 4.36 | 1.16 | 1.72 | −0.1089 | 0.9185 |
| 8B | 2 | mg/kg SM | 100.38 | 1336.17 | 6777.45 | 95,976.0 | −7.7104 | 0.0045 |
| 8D | 2 | µg/L SM | 0.33 | 3.33 | 0.29 | 2.92 | −3.3427 | 0.0288 |
| 8F | 2 | µg/L SM & diss. | 0.54 | 26.10 | 0.46 | 14.69 | −13.133 | 0.0010 |
| Figure | River | R2 | R | t | p |
|---|---|---|---|---|---|
| 4 Top-left | 1 | 0.0011 | 0.0329 | 0.0932 | 0.9280 |
| 4 Top-right | 2 | 0.4565 | 0.6756 | 2.5920 | 0.0320 |
| 4 Bottom-left | 1 | <0.0001 | −0.0049 | −0.0137 | 0.9894 |
| 4 Bottom-right | 2 | 0.4327 | −0.6578 | −2.4702 | 0.0387 |
| 6 Left | 1 | 0.2552 | 0.5052 | 1.6558 | 0.1364 |
| 6 Right | 2 | 0.0881 | 0.2968 | 0.8791 | 0.4050 |
| CW | No. of Samples | Summation | PBTC | HEDP | ATMP | EDTMP | DTPMP | |
|---|---|---|---|---|---|---|---|---|
| mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | |||
| 31 | 10 before WWTP | 18.00 | 7.73 | 8.84 | 0.41 | <LOQ | 1.02 | Mean |
| - | 45 | 92 | 59 | - | 63 | CV/(%) | ||
| 10 behind WWTP | 21.98 | 7.45 | 12.83 | 0.53 | <LOQ | 1.17 | Mean | |
| - | 49 | 34 | 72 | - | 64 | CV/(%) | ||
| Significance of increase in total phosphonate: t = −0.8676, p = 0.3985 | ||||||||
| 40 | 5 before WWTP | 6.66 | 4.19 | 1.96 | 0.15 | <LOQ | 0.36 | Mean |
| - | 54 | 73 | 68 | - | 91 | CV/(%) | ||
| 6 behind WWTP | 17.80 | 10.31 | 6.13 | 0.40 | <LOQ | 0.96 | Mean | |
| - | 14 | 28 | 28 | - | 27 | CV/(%) | ||
| Significance of increase in total phosphonate: t = −5.3294, p = 0.0011 | ||||||||
| 47 | 5 before WWTP | 14.24 | 9.01 | 4.14 | 0.39 | <LOQ | 0.70 | Mean |
| - | 34 | 41 | 37 | - | 45 | CV/(%) | ||
| 6 behind WWTP | 21.77 | 12.59 | 7.63 | 0.47 | 0.12 | 0.96 | Mean | |
| - | 37 | 30 | 42 | 44 | 34 | CV/(%) | ||
| Significance of increase in total phosphonate: t = −1.9756, p = 0.0796 | ||||||||
| 07 | 6 before WWTP | 29.42 | 14.40 | 12.86 | 0.56 | 0.16 | 1.44 | Mean |
| - | 75 | 74 | 68 | 28 | 76 | CV/(%) | ||
| 5 behind WWTP | 32.17 | 15.73 | 13.80 | 0.57 | 0.11 | 1.96 | Mean | |
| - | 21 | 23 | 22 | 24 | 21 | CV/(%) | ||
| Significance of increase in total phosphonate: t = −0.2983, p = 0.7756 | ||||||||
References
- European Phosphonate Association. Phosphonates in Detergents; EPA Detergent Phosphonates Dossier: Brussels, Belgium, 2013. [Google Scholar]
- Rott, E.; Steinmetz, H.; Metzger, J.W. Organophosphonates: A review on environmental relevance, biodegradability and removal in wastewater treatment plants. Sci. Total Environ. 2018, 615, 1176–1191. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; Stone, A.T. Degradation of Nitrilotris(methylenephosphonic Acid) and Related (Amino)Phosphonate Chelating Agents in the Presence of Manganese and Molecular Oxygen. Environ. Sci. Technol. 2000, 34, 4759–4765. [Google Scholar] [CrossRef]
- Matthijs, E.; de Oude, N.T.; Bolte, M.; Lemaire, J. Photodegradation of ferric ethylenediaminetetra(methylenephosphonic acid) (EDTMP) in aqueous solution. Water Res. 1989, 23, 845–851. [Google Scholar] [CrossRef]
- Schowanek, D.; Verstraete, W. Phosphonate utilization by bacterial cultures and enrichments from environmental samples. Appl. Environ. Microbiol. 1990, 56, 895–903. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.K.; Raue, B.; Brauch, H.-J.; Sacher, F. Trace-level analysis of phosphonates in environmental waters by ion chromatography and inductively coupled plasma mass spectrometry. Int. J. Environ. Anal. Chem. 2014, 94, 385–398. [Google Scholar] [CrossRef]
- Müller, U.; Sacher, F. Effects of Drinking Water Softening Concentrates in Flowing Waters (Auswirkungen von Konzentraten der Trinkwasserenthärtung in Fließgewässern); Final report project: 333/2012 No. 71; TZW, DVGW Water Technology Center: Karlsruhe, Germany, 2016. [Google Scholar]
- Wang, S.; Sun, S.; Shan, C.; Pan, B. Analysis of trace phosphonates in authentic water samples by pre-methylation and LC-Orbitrap MS/MS. Water Res. 2019, 161, 78–88. [Google Scholar] [CrossRef]
- Fischer, K. Sorption of chelating agents (HEDP and NTA) onto mineral phases and sediments in aquatic model systems: Part I: Sorption onto clay minerals. Chemosphere 1991, 22, 15–27. [Google Scholar] [CrossRef]
- Fischer, K. Distribution and elimination of HEDP in aquatic test systems. Water Res. 1993, 27, 485–493. [Google Scholar] [CrossRef]
- Rott, E.; Nouri, M.; Meyer, C.; Minke, R.; Schneider, M.; Mandel, K.; Drenkova-Tuhtan, A. Removal of phosphonates from synthetic and industrial wastewater with reusable magnetic adsorbent particles. Water Res. 2018, 145, 608–617. [Google Scholar] [CrossRef]
- Rott, E.; Happel, O.; Armbruster, D.; Minke, R. Behavior of PBTC, HEDP, and Aminophosphonates in the Process of Wastewater Treatment. Water 2020, 12, 53. [Google Scholar] [CrossRef] [Green Version]
- Armbruster, D.; Rott, E.; Minke, R.; Happel, O. Trace-level determination of phosphonates in liquid and solid phase of wastewater and environmental samples by IC-ESI-MS/MS. Anal. Bioanal. Chem. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Fürhacker, M.; Lesueur, C.; Pfeffer, M.; Köllensperger, G.; Popp, M.; Mentler, A. Phosphonates—AMPA (Aminomethylphosphonic Acid). Assessing Origin, Environmental Concentrations and Photolysis Degradation (Phosphonate—AMPA (Aminomethylphosphonsäure). Herkunftsabschätzung, Umweltkonzentrationen und Photolyseabbau); Final report (project no. 1378); Institute of Sanitary Engineering and Water Pollution Control: Vienna, Austria, 2005. [Google Scholar]
- Launay, M.A.; Dittmer, U.; Steinmetz, H. Organic micropollutants discharged by combined sewer overflows—Characterisation of pollutant sources and stormwater-related processes. Water Res. 2016, 104, 82–92. [Google Scholar] [CrossRef]
- HERA. Human & Environmental Risk Assessment on Ingredients of European Household Cleaning Products. Phosphonates; (CAS 6419-19-8; 2809-21-4; 15827-60-8); HERA: Brussels, Belgium, 2004. [Google Scholar]
- Bordas, F.; Bourg, A.C.M. Effect of Complexing Agents (EDTA and ATMP) on the Remobilization of Heavy Metals from a Polluted River Sediment. Aquat. Geochem. 1998, 4, 201–214. [Google Scholar] [CrossRef]
- Gledhill, W.E.; Feijtel, T.C.J. Environmental Properties and Safety Assessment of Organic Phosphonates Used for Detergent and Water Treatment Applications. In Detergents; Oude, N.T., Ed.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 261–285. ISBN 978-3-662-14983-6. [Google Scholar]
- Günther, K.; Henze, W.; Umland, F. Mobilization behavior of thallium and cadmium in a river sediment (Mobilisationsverhalten von Thallium und Cadmium in einem Flußsediment). Anal. Bioanal. Chem. 1987, 327, 301–303. [Google Scholar] [CrossRef]
- Knepper, T.P.; Weil, H. Study on the Entry of Synthetic Chelating Agents and Compounds Exhibiting Complexing Properties into the Aquatic Environment (Studie zum Eintrag synthetischer Komplexbildner und Substanzen mit komplexbildenden Eigenschaften in die Gewässer). Vom Wasser 2001, 97, 193–232. [Google Scholar]
- Müller, G.; Steber, J.; Waldhoff, H. On the influence of hydroxyethanediphosphonic acid on phosphate elimination with FeCl3 and the remobilization of heavy metals: Results of laboratory and field tests (Zum Einfluß von Hydroxyethandiphosphonsäure auf die Phosphatelimination mit FeCl3 und die Remobilisierung von Schwermetallen: Ergebnisse von Labor- und Feldversuchen). Vom Wasser 1984, 63, 63–78. [Google Scholar]
- Jaworska, J.; van Genderen-Takken, H.; Hanstveit, A.; van de Plassche, E.; Feijtel, T. Environmental risk assessment of phosphonates, used in domestic laundry and cleaning agents in the Netherlands. Chemosphere 2002, 47, 655–665. [Google Scholar] [CrossRef]
- Armbruster, D.; Müller, U.; Happel, O. Characterization of phosphonate-based antiscalants used in drinking water treatment plants by anion-exchange chromatography coupled to electrospray ionization time-of-flight mass spectrometry and inductively coupled plasma mass spectrometry. J. Chromatogr. A 2019, 1601, 189–204. [Google Scholar] [CrossRef]
- Forlani, G.; Prearo, V.; Wieczorek, D.; Kafarski, P.; Lipok, J. Phosphonate degradation by Spirulina strains: Cyanobacterial biofilters for the removal of anticorrosive polyphosphonates from wastewater. Enzyme Microb. Technol. 2011, 48, 299–305. [Google Scholar] [CrossRef]
- Raschke, H.; Rast, H.-G.; Kleinstück, R.; Sicius, H.; Wischer, D. Utilization of 2-phosphonobutane-1,2,4-tricarboxylic acid as source of phosphorus by environmental bacterial isolates. Chemosphere 1994, 29, 81–88. [Google Scholar] [CrossRef]
- Rott, E. Investigations on the Elimination of Phosphorus from Phosphonate-Containing Industrial Wastewaters (Untersuchungen zur Elimination von Phosphor aus phosphonathaltigen Industrieabwässern); DIV Deutscher Industrieverlag GmbH: Munich, Germany, 2016; ISBN 978-3-8356-7349-6. [Google Scholar]
- The Control of Eutrophication of Lakes and Reservoirs; Ryding, S.-O.; Rast, W. (Eds.) Unesco: Paris, France, 1989; ISBN 1-85070-257-8. [Google Scholar]
- Schöberl, P.; Huber, L. Ecologically relevant data on non-surfactant ingredients in detergents and cleaners (Ökologisch relevante Daten von nichttensidischen Inhaltsstoffen in Wasch- und Reinigungsmitteln). Tenside Surfact. Det. 1988, 25, 99–107. [Google Scholar]
- Kuhn, R.; Jensch, R.; Bryant, I.M.; Fischer, T.; Liebsch, S.; Martienssen, M. The influence of selected bivalent metal ions on the photolysis of diethylenetriamine penta(methylenephosphonic acid). Chemosphere 2018, 210, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.; Tóth, E.; Geppert, H.; Fischer, T.; Liebsch, S.; Martienssen, M. Identification of the Complete Photodegradation Pathway of Ethylenediaminetetra(methylenephosphonic acid) in Aqueous Solution. Clean Soil Air Water 2017, 45, 1500774. [Google Scholar] [CrossRef]
- Rott, E.; Minke, R.; Bali, U.; Steinmetz, H. Removal of phosphonates from industrial wastewater with UV/FeII, Fenton and UV/Fenton treatment. Water Res. 2017, 122, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B. Environmental chemistry of phosphonates. Water Res. 2003, 37, 2533–2546. [Google Scholar] [CrossRef]
- Zaranyika, M.F.; Nyandoro, M.G. Degradation of glyphosate in the aquatic environment: An enzymic kinetic model that takes into account microbial degradation of both free and colloidal (or sediment) particle adsorbed glyphosate. J. Agric. Food Chem. 1993, 41, 838–842. [Google Scholar] [CrossRef]
- Fischer, K. Sorption of chelating agents (HEDP and NTA) onto mineral phases and sediments in aquatic model systems. Chemosphere 1992, 24, 51–62. [Google Scholar] [CrossRef]
- Ahlgren, J.; Reitzel, K.; Danielsson, R.; Gogoll, A.; Rydin, E. Biogenic phosphorus in oligotrophic mountain lake sediments: Differences in composition measured with NMR spectroscopy. Water Res. 2006, 40, 3705–3712. [Google Scholar] [CrossRef]
- Bai, X.; Ding, S.; Fan, C.; Liu, T.; Shi, D.; Zhang, L. Organic phosphorus species in surface sediments of a large, shallow, eutrophic lake, Lake Taihu, China. Environ. Pollut. 2009, 157, 2507–2513. [Google Scholar] [CrossRef]
- Benitez-Nelson, C.R.; O’Neill, L.; Kolowith, L.C.; Pellechia, P.; Thunell, R. Phosphonates and particulate organic phosphorus cycling in an anoxic marine basin. Limnol. Oceanogr. 2004, 49, 1593–1604. [Google Scholar] [CrossRef] [Green Version]
- Kittredge, J.S.; Roberts, E. A Carbon-Phosphorus Bond in Nature. Science 1969, 164, 37–42. [Google Scholar] [CrossRef] [PubMed]








| River | Phase | Position Related to WWTP | PBTC | HEDP | ATMP | EDTMP | DTPMP |
|---|---|---|---|---|---|---|---|
| River1 | Dissolved | Before | 69.6 | 17.2 | 0.0 | 0.7 | 12.5 |
| Behind | 72.3 | 15.4 | 0.0 | 0.0 | 12.3 | ||
| Sediment | Before | 42.8 | 50.2 | 1.7 | 0.1 | 5.2 | |
| Behind | 37.5 | 55.0 | 1.6 | 0.1 | 5.8 | ||
| Suspended matter | Before | 34.0 | 56.1 | 0.8 | 0.4 | 8.7 | |
| Behind | 29.5 | 59.4 | 1.0 | 0.3 | 9.8 | ||
| River2 | Dissolved | Before | 5.3 | 74.3 | 0.0 | 0.0 | 20.4 |
| Behind | 34.1 | 64.0 | 0.0 | 0.0 | 1.9 | ||
| Sediment | Before | 6.4 | 79.2 | 0.0 | 0.0 | 14.4 | |
| Behind | 20.1 | 71.6 | 1.7 | 0.0 | 6.6 | ||
| Suspended matter | Before | 3.2 | 48.3 | 0.9 | 0.1 | 47.5 | |
| Behind | 12.3 | 82.7 | 2.0 | 0.3 | 2.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rott, E.; Happel, O.; Armbruster, D.; Minke, R. Influence of Wastewater Discharge on the Occurrence of PBTC, HEDP, and Aminophosphonates in Sediment, Suspended Matter, and the Aqueous Phase of Rivers. Water 2020, 12, 803. https://doi.org/10.3390/w12030803
Rott E, Happel O, Armbruster D, Minke R. Influence of Wastewater Discharge on the Occurrence of PBTC, HEDP, and Aminophosphonates in Sediment, Suspended Matter, and the Aqueous Phase of Rivers. Water. 2020; 12(3):803. https://doi.org/10.3390/w12030803
Chicago/Turabian StyleRott, Eduard, Oliver Happel, Dominic Armbruster, and Ralf Minke. 2020. "Influence of Wastewater Discharge on the Occurrence of PBTC, HEDP, and Aminophosphonates in Sediment, Suspended Matter, and the Aqueous Phase of Rivers" Water 12, no. 3: 803. https://doi.org/10.3390/w12030803