Helicoverpa genus on the edge of the continental U.S.: Flight phenology, analysis of hybrid presence, and insecticide performance in high-input field crops in Puerto Rico
- 1Syngenta Seeds, Salinas, Puerto Rico
- 2Entomology & Nematology Department, West Florida Research and Education Center, Jay, FL, United States
- 3Syngenta Crop Protection Inc., Greensboro, NC, United States
- 4Entomology & Nematology Department, University of Florida, Gainesville, FL, United States
- 5Southern Insect Management Research Unit, USDA Agricultural Research Service, Stoneville, MS, United States
The genus Helicoverpa includes several agricultural pests globally. Helicoverpa armigera was reported in several countries in South America in 2013, and in Puerto Rico, in 2014. This territory is considered an agricultural hub, with a high-input system of seed production in the southern region of the island, and also at the edge of the continental U.S. Possible natural dispersion of populations of H. armigera from the Caribbean or other Central American regions poses a continuing risk to the U.S. This study was performed during the post-detection scenario of H. armigera in Puerto Rico, from 2018 to 2021. A year-round pheromone trapping program of adult males indicated an increase in the population from October to March and differences in the occurrence of Helicoverpa spp. between the municipalities Juan Diaz and Salinas. The proportion of H. armigera/H. zea and detection of congeneric hybrids between these species were assessed based on genital morphology and DNA analysis. Interestingly, neither H. armigera nor expected hybrids were detected in the present study. The susceptibility of H. zea populations to the insecticides Spinetoram, Emamectin benzoate, Chlorantraniliprole, and Esfenvalerate was assessed, and an overall significant effect of insecticide susceptibility was detected. Chlorantraniliprole and Emamectin benzoate had the highest efficacy. These results contribute to the Integrated Pest Management and Insect resistance management programs to Helicoverpa spp. in Puerto Rico. In addition, provide validated information to be considered in mitigation plans, in the scenario of an invasion of H. armigera in the continental U.S.
Introduction
A review of the subfamily Heliothinae (Lepidoptera: Noctuidae), a cosmopolitan group of noctuid moths proposed the genus Helicoverpa (1), which differentiated the species H. armigera (Hübner) and H. zea (Boddie) (Lepidoptera: Noctuidae) based on male genitalia morphology. The larvae and adults of both species have similar morphological characteristics and precise identification could only be based on male genitalia dissection (1, 2), and more recently, molecular approaches (3–5).
Previously, these species were allopatric occurring in separate geographical regions, with H. zea considered to be derived from H. armigera due to a genetic bottleneck that occurred 2 million years ago (4, 6). Helicoverpa zea is found throughout the Americas (1). In the United States, more than 30 crops are the host for this species and is considered to be one of the most important pests in row crops, southern U.S., including cotton (7). Helicoverpa armigera is reported to have a broader host range than H. zea (1, 8) and is widespread in Africa, Europe, Asia, and Oceania (9). This species is considered a major pest of food, fiber, and oil crops and has been reported in more than 67 host plant families, including Asteraceae, Fabaceae, Malvaceae, Poaceae, and Solanaceae (8).
Data from 1,208 interceptions of H. armigera in the international trade of commodities from 77 countries, including the Netherlands, Israel, and North Africa reported the association of this pest with food plants and cut flowers (10). Helicoverpa armigera was first reported in Brazil causing outbreaks during the 2012-2013 crop season, in commercial fields of cotton, corn, soybean, tomato, and beans, among other host plants (11, 12). A follow-up study recovering specimens from previous collections in the country and performing genitalia dissections (2), and PCR-RFLP molecular analysis following Behere et al. (3) indicated that this species was already present in the south region of Brazil at least before October 2008 (13). Further detections of this species were reported in Paraguay (14), Argentina (15), Uruguay (16), Colombia, Peru, Surinam, and the Dominican Republic (17).
The potential of invasion of this species in the continental U.S. was modeled and indicated the risk of natural dispersal from Caribbean islands or Mexico, due to the existence of suitable climate and extensive areas of host crop plants, especially in the southern U.S., with an estimated impact of US$ 78 billion p.a (18). In Puerto Rico, H. armigera was reported by the Plant Protection and Quarantine program (PPQ) (19) as a result of the USDA survey effort in 2014 (20). Phylogeographic analyses were performed based on comparing several haplotypes of 171 specimens of H. armigera, from 27 countries (10). These analyses included the three specimens intercepted in 2015 during survey trapping in south Florida (21), one year after detection in Puerto Rico. The results of these analyses could not determine the origin of the H. armigera population detected in both Puerto Rico and the three specimens in Florida (10). A study conducted between February 2016 and January 2017 in Puerto Rico identified four specimens of H. armigera in the sex pheromone trapping (22). However, it was detected at a low occurrence of H. armigera and a high abundance of H. zea, which can suggest that the invasion of this species was in its early stage (23).
Puerto Rico is considered an agricultural hub, with a high-input system of seed production in the southern region of the island, due to the tropical climate that allows extended crop season (24). This region is also on the edge of the area of the continental U.S., which possible natural dispersion of populations of H. armigera from the Caribbean or other Central American regions to the continental U.S. poses a continuing risk. However, few studies have been performed on the populations of the genus Helicoverpa in the post-detection scenario of H. armigera in Puerto Rico (10, 22, 23). More information is needed to support management recommendations for the genus Helicoverpa, including the expected occurrence of hybrids between H. zea and H. armigera. The objectives of this study were: 1) Document the occurrence and seasonal flight of H. armigera in the host plants corn, soybean, and sunflower in the southern region; 2) Determine the possible occurrence of hybrids between H. armigera and H. zea caught in the pheromone trapping; and 3) Evaluate the performance of insecticides commonly adopted to manage this genus in high-input systems of seed production. The results of this work represent a contribution to the improvement of the IPM and Insect Resistance management of the Helicoverpa genus in Puerto Rico, considering the expected coexistence of the native H. zea with H. armigera. This study provides also data on the magnitude of occurrence and seasonal phenology of flight on the island, and the performance of insecticides currently adopted for the management of Helicoverpa spp. The results of this work also contribute with validated information for mitigation plans, in a scenario of H. armigera invasion in the continental U.S.
Materials and methods
Year-round trapping of Helicoverpa sp. in commercial fields
A continuous trapping program for H. armigera and H. zea was conducted from 2018 to 2021 in the municipalities of Salinas (17° 58’38.89” N, -66° 17’52.62” W) and Juana Díaz (18°03’8.86N, -66°30’23.62W) in Puerto Rico. These two locations were selected for the year-round pheromone trapping because this southern region of Puerto Rico concentrates a large production of vegetables and is one of the largest winter nursery breeding operations in the United States of soybean, sunflower, corn, cotton, and sorghum (24). A total of ten bucket traps were placed in commercial fields of corn, soybean, and sunflower, and 19 traps in open field areas during the fallow season, using green bucket traps (International Pheromone Systems, IPS, Vestaburg, MI), with the H. armigera sex ABW pheromone lure (Trece, Inc., Adair, OK). The field sizes vary from 0.5 to 2 acres and the criterion of one trap in average per acre was followed, in a total of five bucket traps placed in corn, two bucket traps in soybean, and three bucket traps in sunflower fields. In addition, six Texas cone traps (25, 26), and 13 Scentry Heliothis traps (Scentry Biologicals, Inc., Billings, MT) were set in the fields with the H. zea L215 sex pheromone lure (Scentry Biologicals, Inc., Billings, MT). Traps were positioned at least 100 m apart. Bucket traps were mounted around a 1.2 m above-ground wood stake, on the east edge side of each field (wind direction is southwest) to promote dispersion of pheromone scent into the fields. Pheromone lures were replaced every 3 weeks. Moth samples captured in the traps were collected weekly, stored in 26.8cm x 27.3cm Ziploc® plastic bags (S. C. Johnson, Racine, WI), transported to the Syngenta Seed Production System Laboratory in Salinas, PR, and kept in an upright freezer (-18°C) pending subsequent genitalia dissection and molecular analysis for species identification.
Helicoverpa spp. identification
An initial sample screening was performed for each pheromone trap collection to separate specimens of Helicoverpa spp. from other cross-attracted species. Moths of the genus Helicoverpa were identified based on the presence of the wing morphological characteristics, such as a black color spot on the forewing, the presence of a broad dark transverse band distally, and hind wings lighter in color (1). A subsample of 76 specimens of Helicoverpa spp. collected from corn fields in Juana Díaz were then dissected for identification based on male genitalia morphology (2). The criteria for selection of the subsamples were based on morphological characteristics and the moths were collected from traps placed near corn fields in the reproductive stage (R1-R2). Each specimen was identified by a code, and the abdomen of the subsample of the Helicoverpa moths was removed using forceps and placed in 70% isopropyl alcohol for approximately 2 minutes to re-hydrate, before transferring to an individual 20mL-glass vial filled with 10 mL of a 10% potassium hydroxide solution (10% KOH). The abdomens were heated to 50°C for 45 minutes. After 45 minutes the KOH was removed using a dropper, and the abdomens were rinsed with alcohol (Brambila 2009). The abdomen was placed in a petri dish using a 7X-45X Simul-Focal Trinocular Zoom Stereo Microscope (AMScope, Irvine, CA, USA) and the male genitalia was extruded, using a fine point, straight tip, stainless forceps (BioQuip, Rancho Dominquez, CA, USA), applying light pressure from the base to the apex of the abdomen to extrude the genitalia. A fine paintbrush (Walmart®, Santa Isabel, PR) was used to brush clean the aedeagus before looking at the diagnostic characteristics. The diagnostic genitalia characteristic used for the identification of Helicoverpa spp. followed Pogue (2), considering the number of small lobes at the base of the vesica, near the apex of the aedeagus. A specimen with three lobes was identified as H. zea, and a specimen with a single lobe was identified as H. armigera, following Pogue (2).
To determine the genetics of the Helicoverpa spp. samples were collected, a subsample of 550 specimens was selected and DNA analysis was performed in the Entomology Laboratory at West Florida Research and Education Center, Jay, Florida. DNA was extracted from individual moths following the manufacturer’s instructions, using Qiagen Blood and tissue kit (cat. #65506). The PCR-based method was used for species identification of H. zea and H. armigera using the three-primer cocktail high-resolution melt curve (HRM) method developed by Perera et al. (5). DNA samples were subjected to PCR amplification with the three-primer cocktail and the amplicons were resolved on a 1% agarose gel. Six specimens with two amplicons were tentatively designated as hybrids and were submitted to sequence analysis in the Southern Insect Management Research Unit, USDA, ARS, Stoneville, MS. Ribosomal RNA gene region of approximately 1300 bp containing the internal transcribed spacer (ITS) 1, 5.8S rRNA subunit, and ITS2 was amplified from DNA extracted from the putative hybrid insects and control H. zea using a forward primer designed to 18S (310; 5’- ATCATTTAGAGGAAGTAAAAGTCGTAACAAGGT -3’) and a reverse primer designed to 28S (387; 5’- TTCCTGTTCGCTCGCCGCTACT-3’). Amplicons were resolved on a 0.8% agarose gen and the DNA bands excised from the gel were purified using QiaX gel purification reagents following the manufacturer’s instructions (Qiagen). The resulting DNA fragments were cloned into PCR2.1 vector using TOPO TA cloning kit (Invitrogen) and 12 recombinant colonies representing each insect were submitted to USDA ARS Genomics and Bioinformatics Research Unit, Stoneville, MS for Sanger dideoxy sequencing. ITS 1 and ITS2 nucleotide sequences from the suspicious hybrids from the PCR-based method and control insects were aligned with the respective sequences from H. zea and H. armigera to determine the source species.
Insecticide susceptibility bioassays
The insecticide susceptibility of populations of the Helicoverpa genus to pyrethroid, avermectin, spinosyn, and diamide were documented in populations collected in corn ears, in Salinas, and in Juana Diaz. Around 150 larvae per location, ranging from 2nd to 4th instars were collected to establish field-derived colonies to be used in bioassays. The bioassays were conducted in the Syngenta Seed Production System Laboratory in Salinas, PR. Larvae were collected from corn fields in each municipality (trap crop and field corn) during the 2021 crop season. Larvae of Helicoverpa spp. were identified based on the presence of spines on the body of the larvae to distinguish this genus from Spodoptera frugiperda, another prevalent species associated with corn in the region. In addition, a subsample of 10 insects from the field-derived colonies from each municipality was submitted to DNA analysis as previously described for species identification and validation of the species identity of the colonies. The field-derived colony was established by placing each larva collected in field in a 71 grams souffle plastic cup containing an all-purpose Lepidoptera diet (Frontier Agricultural Sciences, Newark, DE) and transported to the seed production system laboratory in Salinas. Larvae were maintained at 25 ± 1°C, 40% relative humidity, and 12 h:12 h, light: dark photoperiod. The pupae were placed in Petri dishes inside 3.8-liter plastic containers (ePackageSupply, Evansville, IN) and used as mating cages. White cotton cloth was placed at the top of the container to serve as an oviposition surface. The moths were fed with a 20% sucrose solution change every two days. The egg sheets were collected daily and placed in a 9.4-liter rectangular plastic container (Rubbermaid food storage container). Approximately 200 neonates were transferred to 71 grams souffle plastic cups with an all-purpose Lepidoptera diet from each population and reared until they reached 3rd instar and had the appropriate size for the performance of the bioassays.
The bioassays with esfenvalerate, emamectin benzoate, and spinetoram were performed following Da Silva et al. (23), in which bioassay cups on 30-well trays were filled with 1 mL of artificial moth diet per well and insecticides dilutions applied on the diet surface. After 30 minutes, one single larva was placed inside the well
Diet overlay bioassays using 128-well trays (Frontier Agricultural Sciences, Newark, DE) were filled with 1 mL of general-purpose lepidopteran diet (Frontier Agricultural Sciences, Newark, DE). Once the diet was solidified and cool, 20µL of the insecticide concentration with 5% of a surfactant was dispensed on top of the diet, covering the entire surface of the 1.5 cm2 well. After the solution had dried, a single third instar Helicoverpa spp. larva was placed on top of the diet using a fine touch painting brush (Walmart®, Santa Isabel, PR).
Insecticide dilutions of insecticides registered to manage Helicoverpa sp. in the region were selected for this study. The high label rate per acre of commercial formulations of the insecticides Esfenvalerate (Asana® XL, Valent®), Emamectin benzoate (Proclaim®, Syngenta®), Spinetoram (Radiant® SC, Corteva Agriscience™) (27), and Chlorantraniliprole (Coragen®, FMC Ag US), were prepared in distilled water with adjuvants (Table 1). Four repetitions of 12 larvae for each product rate were prepared, plus a control group that consisted of a general-purpose diet and a solution of distilled water with a 5% surfactant. Larval mortality was assessed at 48 h and the number of dead larvae was recorded. The bioassay with chlorantraniliprole followed the Insecticide Resistance Action Committee (28) Method Number 20, as is recommended by the IRAC Diamide Working Group for evaluating the susceptibility status of diamides insecticides. The 128-well bioassay trays (Frontier Agricultural Sciences) were filled with 1g of stonefly Heliothis premix diet (Educational Science, League City, TX) mixed with the insecticide dilution and surfactant. The number of replications and quantity of larvae for the diet incorporated bioassay were the same as for the diet overlay bioassay previously described. Daily inspections indicated that 100% of larvae were dead after 48 h of exposure, but final larval mortality was assessed on day 7.

Table 1 Commercial insecticides and label rate tested in susceptibility bioassays to document Helicoverpa sp. populations from Salinas and Juana Diaz, Puerto Rico. 2021 crop season.
Statistical analysis
The residual plots in SAS were used to test the best fit of the data distribution of the pheromone trapping data, and the Poisson distribution provided a better fit. The effect of the municipality and month in the total number of Helicoverpa spp. moth caught were analyzed using a Generalized linear mixed model (GLMM) on SAS (version 9.4). Due to the nested nature of the pheromone trapping, crop and location were included in the model as nested random effects. A significant interaction was detected between the trapping month and the municipality, the means were compared using the Tukey’s HSD test (p-value= 0.05). The percentage mortality for each insecticide was calculated and corrected considering the mortality of control in each bioassay, following Abbotts’ formula. Differences in the insecticide susceptibility of Helicoverpa sp. populations were tested using LS Means’ S test (p-value = 0.05).
Results
Year-round trapping of Helicoverpa sp. in commercial fields
The continuous pheromone trapping of Helicoverpa spp. in high-input systems cultivated with corn, soybean, and sunflower in the municipalities of Salinas (Figure 1A) and Juana Díaz (Figure 1B) in Puerto Rico indicated flight throughout the year, with a high occurrence of Helicoverpa spp. moths from October to March. Between the 2018 to 2021 crop seasons, a total of 1,835 moths were caught in the trapping with H. armigera pheromone lure in Juana Díaz, and 734 moths were caught in the municipality of Salinas. The trapping performed with H. zea pheromone lure indicated the same pattern of a flight of Helicoverpa spp. in the regions under study (Figures 2A, B). A total of 5,998 and 1,123 H. zea moths were caught in Juana Díaz and in Salinas, respectively. The effect of the municipality (Salinas and Juana Diaz), crop, and month on the abundance of Helicoverpa spp. were tested, and a significant interaction between municipality and month was detected (p-value <0.0001; F-Value=92.23). During the months from September to February, a high abundance of Helicoverpa spp. was detected in Juana Diaz (Table 2). Conversely, a high abundance of Helicoverpa spp. was detected in Salinas, during March, April, and May (Table 2). Morphological dissections of the male genitalia of a subsample from the trapping with H. armigera lure indicated that all the specimens had the presence of the three lobes at the base of the vesica and were identified as H. zea. The samples were analyzed using the HRM method validated by Perera et al. (5) initially indicated the possible existence of six hybrids of H. armigera x H. zea collected from corn fields and fallow area in Juana Diaz, and all remaining analyzed specimens were identified as H. zea. Subsequent nucleotide sequence analysis of ITS1 and ITS2 regions did not confirm the presence of H. armigera x H. zea hybrids in the collections, indicating that hybrids were not detected in any collection site.
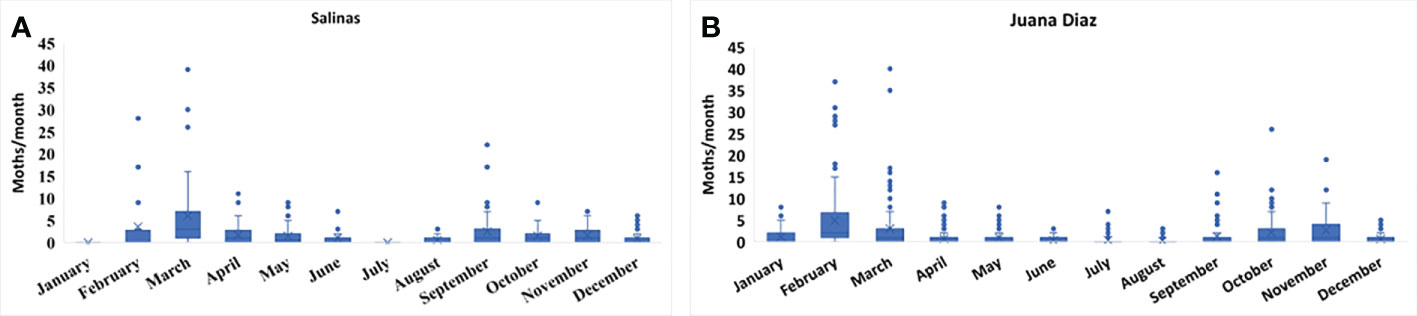
Figure 1 Total number of moths of Helicoverpa spp. in sex pheromone traps using H. armigera lure from 2018 to 2021. A: Collection in the municipality of Salinas, Puerto Rico. B: Collection in the municipality of Juana Diaz, Puerto Rico.
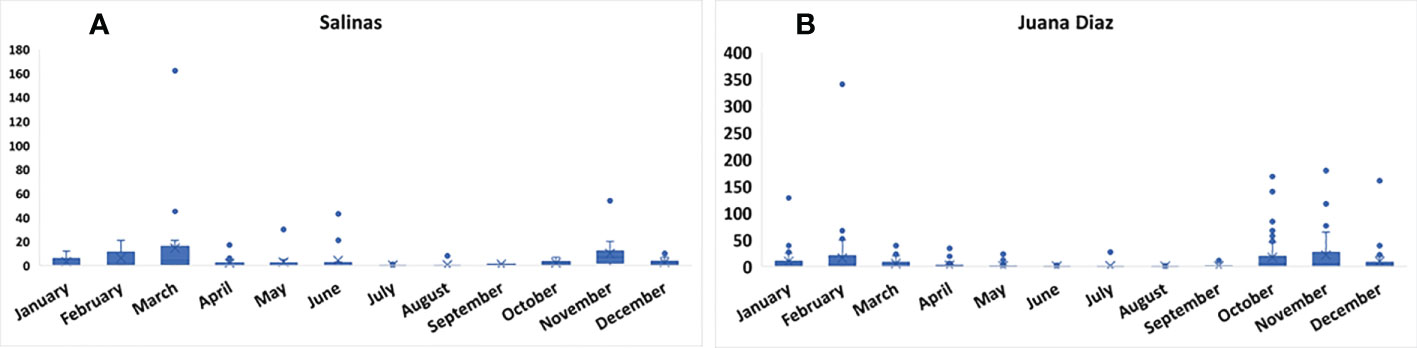
Figure 2 Total number of moths of Helicoverpa spp. in sex pheromone traps using H. zea lure from 2018 to 2021. A: Collection in the municipality of Salinas, Puerto Rico. B: Collection in the municipality of Juana Diaz, Puerto Rico.
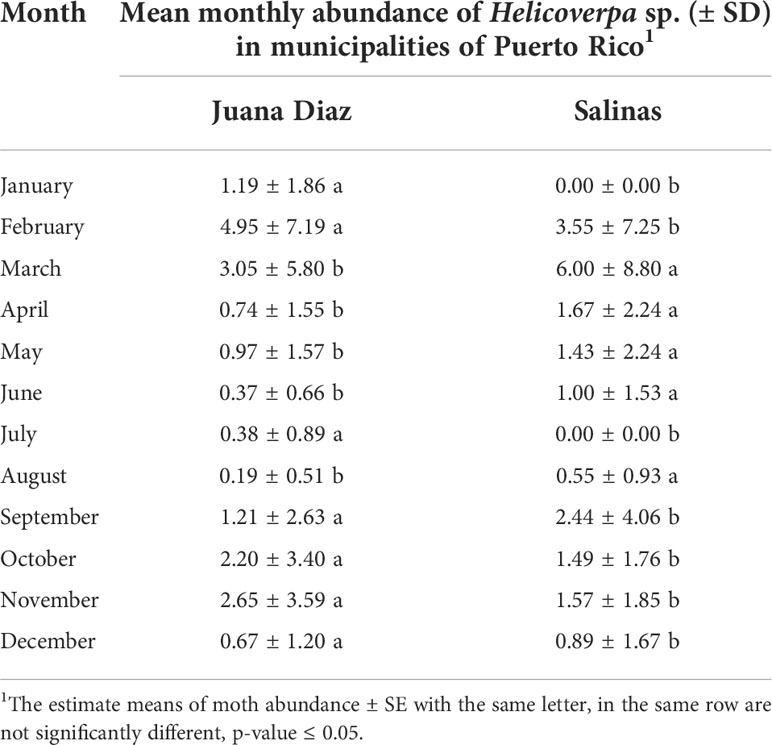
Table 2 Simple effect comparisons of municipality and month on the abundance of Helicoverpa spp. caught in sex pheromone trapping using H. armigera lure in Salinas and Juana Diaz municipalities, Puerto Rico, from 2018 to 2021.
Helicoverpa spp. identification
The PCR analysis of a subsample of insects from the field-derived colonies established in the laboratory from each municipality indicated the presence of only H. zea, which is consistent with the results from the sex pheromone trapping without the presence of hybrids.
Insecticide susceptibility bioassays
The results of bioassays are interpreted as the susceptibility of Helicoverpa spp. populations. The populations from Juana Díaz and Salinas did not differ in insecticide susceptibility (Table 3). However, an overall significant effect of insecticide susceptibility was detected (p-value<0.001, F=87.20) (Table 3). Chlorantraniliprole and emamectin benzoate had statistically similar performance, with mortality higher than 90% (Table 4), followed by spinetoram with mortality above 70%, and esfenvalerate, which had the lowest performance when compared with the other three insecticides (Table 4).
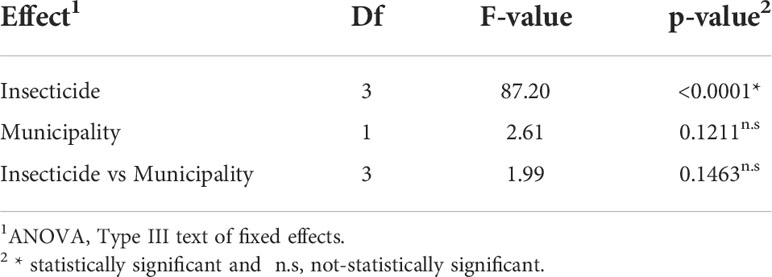
Table 3 Effect of municipality and insecticide in the susceptibility of populations of Helicoverpa spp. at label rate recommendation.
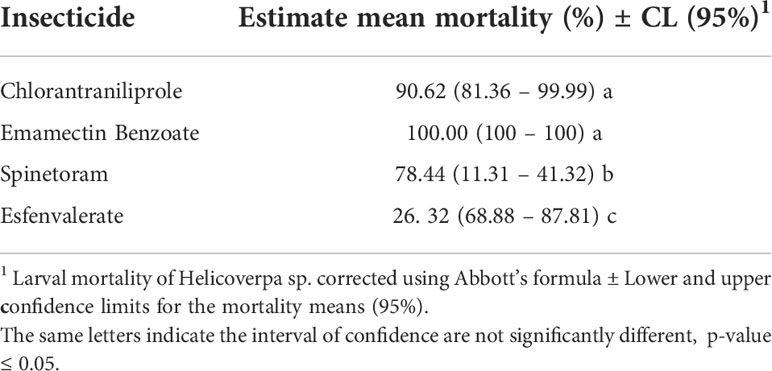
Table 4 Performance of Helicoverpa spp. populations at label rate concentrations of predominant insecticides adopted in high-input systems of field crop production in Puerto Rico.
Discussion
Trapping Helicoverpa spp. using sex pheromone lures over three years in high-input production systems of field crops, indicates the existence of year-round flight of this genus in Puerto Rico. A seasonal flight trend was also documented, with overall high moth abundance from October to March, and low abundance from April to September. However, even though the moths of this genus are strong fliers and able to spread throughout different regions (29), differences in moth abundance of Helicoverpa spp. between the municipalities in different months were significant. During the main crop season in Puerto Rico, which ranges from October to March, Juana Díaz had the highest number of moths caught through pheromone trapping. The municipality of Juana Diaz has one of the largest nursery breeding operations during what is defined as a winter season, from October to March, with high-input systems prevalently cultivated with corn. The agricultural landscape has also soybean, sunflower, and small acreages of sorghum (24). During the fallow season, which is usually from April to September, even though the landscape still has fields of soybean, sunflower, and tomato, the cultivated acreage drops drastically.
In polyphagous species, such as the genus Helicoverpa, the availability of different host plants has an important role in the increase of populations. In the United States, H. zea has a long history as an economic pest of cotton, but corn is the preferred agricultural host (30) together with sorghum, which also supports large populations of H. zea (31). In wild hosts, H. zea is associated with winter vetch, cranesbill, and crimson clover, and in Mississippi, these host plants are described as important early-season hosts. In North Carolina, toadflax and deergrass are reported as alternative hosts of H. zea (32, 33). Helicoverpa armigera also has a wide host plant range, including cultivated and wild plants, with over 172 and 200 host plants in Australia and China, respectively. In Japan, cotton (34, 35) and okra are recorded as top crops for H. armigera (35). In Brazil, in a study conducted post-invasion of H. armigera, the highest survival rates were recorded in soybean, cotton, and cowpea under laboratory conditions (36). The authors hypothesized that cultural practices create a shifting mosaic of habitats, in which populations of H. armigera can create a bridge using uncultivated crops during the fallow season (36), and survived in alternative cultivated hosts, such as green beans, tomatoes, citrus, and pastures (37). A high abundance of moths in Salinas was detected at the beginning of the fallow season, until June. In this municipality, during the crop season, the predominant crop is soybean, surrounded by other local farms, which entire operation are banana trees and other nursery small fields of soybeans and corn. During the fallow season, the region is predominantly cultivated with bananas, plantains, and scattered fields of sorghum. In addition, a remarkable presence of wild cotton is found around the perimeter of the cultivated fields, which during the fallow season is in the reproductive stage. The presence of this native cotton around the field during the autumn season may contribute to the maintenance of a higher abundance of Helicoverpa spp. detected during the pheromone trapping.
The results of male genitalia dissection and DNA analysis indicated only the presence of H. zea in the municipalities under study, even when the trapping used H. armigera pheromone lure. Closely related species of the genus Helicoverpa usually share common sex pheromone components with different ratios, and moths rely on these variations in multi-component pheromones to maintain reproductive isolation (38). A study conducted by Guerrero et al. (39) in two fields in Northern Florida from 2010 to 2011 showed that H. zea and H. armigera rubber septa lure with the same components (Suterra LLC, Bend, Oregon and Tréce, Inc., Adair, Oklahoma) resulted in 11,600 specimens collected and all specimens collected were identified as H. zea by male genitalia dissection. Cross-attraction has been documented in other species of lepidopteran pests, such as in the Plusiinae family, where the commercial formulation lure for Chrysodeixis includens also attracted Ctenoplusia oxygramma, Rachiplusia ou, Trichoplusia ni, and Autographa verruca (40).
In the present study, H. armigera was not detected in the samples of specimens that underwent genitalia dissection and molecular analysis. This result indicates the predominance of native H. zea in the landscape in the two municipalities under study. Previously, the documentation of the interaction and predominance of H. zea and the new invasive H. armigera in Brazil indicated that the proportion of each species was variable in different regions, and possibly due to differences in the agricultural landscapes (41). The aggressive behavior of H. zea when in intraspecific interaction with H. armigera and the variable acreages of corn and cotton were indicated as factors playing a role in the predominance of H. zea or H. armigera in different landscapes, respectively (41). In Puerto Rico, corn is the crop with large, cultivated acreage and may be favoring H. zea.
The genetic analysis for 550 specimens did not detect the presence of hybrids of H. zea and H. armigera in the pheromone trap collections from 2018 to 2021, providing no evidence for the expected field hybridization of these two species in Puerto Rico. Previously, the occurrence of hybridization between these two species was documented in the laboratory, under artificial conditions (1, 42). More recently, the possible interbreeding between H. zea and H. armigera has been indicated, based on the interspecific gene flow after H. armigera invasion in Brazil (43). The propensity of hybridization between these two species was later documented using whole-genome resequencing in combined samples from 16 countries, including specimens from Old World and South American populations (44). In addition, analysis with populations from high-input systems of field crops in Brazil estimated a range of 15 to 30% hybrid occurrence in the samples under analysis (45). The presence of natural hybridizations between H. zea and H. armigera in Puerto Rico is something expected due to the report of the invasive H. armigera in 2014. However, the population size of H. armigera in Puerto Rico is considered negligible (22) and possibly still not established (23), if compared to that in Brazil (46).
The relevance of analysis to detect hybridization between H. zea and H. armigera is due to the probability of new formations of adaptive genes (44, 47), which can have an impact on ecological attributes of the hybrids, such as a wider host range (48, 49) and performance of management tools (28, 50, 51). However, a study documenting biological parameters of hybrids between H. armigera and H. zea, under laboratory conditions, indicated that egg viability is a critical factor for the success of the hybridization, with overall egg viability of 14% (52), when compared with more than 85% egg viability in the parental species. The authors concluded that there are reproductive limitations for hybridization, including barriers from the lock-and-key mechanisms presented in the noctuid genitalia morphology (53, 54). It is expected that hybridization increases through both species’ abundance and time. The first report of H. armigera in Puerto Rico was in 2014, and the DNA analysis performed with samples from 2018 to 2021 did not detect hybrids in moths caught during the pheromone trapping. Future studies should keep monitoring for the expected presence of hybrids in Puerto Rico, especially focusing on specimens collected from corn fields, which is the predominant host crop in the agricultural landscape and represents an appropriate environment for intraguild interactions of both species (41).
The management of Helicoverpa spp. in Puerto Rico relies on larval scouting, moth trapping, crop rotation, the use of biopesticides, natural enemies, and insecticides. Due to the high pest pressure of lepidopteran pests, including Helicoverpa sp., there is a constant selection pressure for insecticide-resistant populations in Puerto Rico. During the crop season, the high-input systems of nursery production in Puerto Rico may require up to 30 insecticide applications in a 70 to 90-day crop cycle. The monitoring of the performance of insecticides with a different mode of action represents a critical aspect in an Insect Resistance Management (IRM) program, in an area-wide approach for the region, which has been implemented after failures to control lepidopteran pests in corn and soybean in Puerto Rico seed nurseries (55). The IRM program includes an approach of bi-monthly rotating insecticide modes of action, and then monthly rotations of insecticides to improve management (55). While this approach targeted pest species other than Helicoverpa sp., it effectively managed Helicoverpa populations at the time of application. The performance of four insecticides commonly adopted in this area wide IRM approach in Puerto Rico was tested considering 48 h of exposure in toxicological bioassays with larval mortality rate. The insecticides tested included chlorantraniliprole, which is a diamide (Coragen®, DuPont™) insecticide. This insecticide is a ryanodine receptor modulator causing contraction and paralysis in targeted pests (56). Emamectin benzoate (Proclaim®, Syngenta®) is an avermectin insecticide and acts as a glutamate-gated chloride channel allosteric modulator that causes paralysis (57). Spinetoram (Radiant® SC, Corteva agriscience™) (27) is a spinosyn insecticide and is a nicotinic acetylcholine receptor allosteric modulator that causes hyperexcitation in the nervous system (Dow AgroSciences, 2006), and Esfenvalerate (Asana® XL, Valent®) is a pyrethroid that acts as a sodium channel modulator (58)
The results of the performance of chlorantraniliprole, emamectin benzoate, spinetoram, and esfenvalerate indicated that there were no differences in the insecticide susceptibilities between populations collected in the two municipalities. The management approach adopted in both municipalities results in the same selection pressure in populations of Helicoverpa spp. However, significantly different performance of insecticides was detected, with the insecticides emamectin benzoate and chlorantraniliprole having a level of control above 90%. These insecticides should be considered in a rotation of mode of action program and efforts to keep tracking performance and susceptibility of populations of genus Helicoverpa in Puerto Rico. Spinetoram provided control below 70%, and esfenvalerate had lower efficacy in comparison to the other insecticides tested in H. zea with 30% or less larval mortality in populations of both municipalities. The use of spinosyns (Spinetoram and Spinosad) to manage Helicoverpa spp. has increased in recent years, with spinetoram being highly toxic to both species (23). In bioassays conducted by da Silva et al. (23), Spinetoram was highly toxic for both Helicoverpa species, H. zea and H. armigera, with spinetoram having an LC50 of 0.11-0.08 ug a.i./cm2. In the present study, spinetoram performance demonstrated a significantly lower performance when contrasted with emamectin benzoate and chlorantraniliprole. In addition, esfenvalerate had the lower performance among the four tested insecticides.
Resistance to pyrethroids in H. zea has been reported in Indiana and Illinois populations, along with others in the Midwest, northcentral, and northeastern populations (59), and more recently in the Florida Panhandle (60). Pyrethroid resistance in H. armigera has also been recorded in Benin and other West African countries (61). Increased cases of pyrethroid resistance in lepidopteran pest populations may lead to high adoption and consequently selection pressure of resistance to diamide insecticides (62). Diamides are relatively safe, and their biological, ecological, and toxicological attributes have high importance in an IRM program (62). The performance of the insecticides tested in this study, based on 48 h mortality with exposure to field rate diagnostic doses, indicated that three of the four insecticides commonly adopted in Puerto Rico should continue to be considered in a rotation to manage Helicoverpa spp. populations in field crop production in Puerto Rico.
In summary, the present study, performed in high-input nursery production of field crops in Puerto Rico, contributes to the IPM and IRM programs. The documentation of the phenology of flight of Helicoverpa spp. in high-input systems indicated that scouting activities in the fields should begin as early as October during the crop season. In addition, cultural control should be considered during the following season to eliminate volunteer plants or alternative hosts of H. zea, such as weeds, which may play a role in Helicoverpa spp. source of infestation during the crop season. The results of the bioassays indicated the differential performance of insecticides, which should be taken into consideration when selecting modes of action for rotation, in an IRM program. In addition, H. zea was the species detected in this study in commercial fields. However, there is a risk of the occurrence of hybrids with H. armigera, especially considering the possible increase of this invasive species, which can represent a challenge since management tools may perform differently. The occurrence of hybrids in the population along with the occurrence of H. armigera should be kept monitored, using updated molecular tools. Overall, the results of this work contribute with information to mitigation plans, in a scenario of an invasion of H. armigera in the continental U.S.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
All authors have contributed equally within specific sections of this manuscript. XLFR, SVPM, and JWJ contributed to the research design. XLFR conducted the experiments, and analyze the pheromone trapping, dissection, and bioassays data along with SVPM. OPP performed the molecular analysis. XLFR and SVPM wrote the manuscript, and JWJ and CJJ did the first English review, and all co-authors read and approved the manuscript.
Funding
The publication fees will were supported by funds from NIFA USDA Hatch Project FLA-WFC-006203, Hatch Multistate project NC246 n. FLA-WFC-006003, and Multistate project S1080 FLA-WFC-006204.
Acknowledgments
Syngenta Seeds Puerto Rico for supporting the master’s program of the first author and the pheromone trapping data collection. Puerto Rico Agricultural Biotechnology Industry Association (PRABIA) for funds for molecular analysis. NIFA USDA Hatch Project FLA-WFC-006203, Hatch Multistate project NC246 n. FLA-WFC-006003, and Multistate project S1080 FLA-WFC-006204 partially funding supplies, and publications fees. Izailda B. dos Santos and Kelsey Hope for DNA extraction and analysis (UF/IFAS/West Florida Research Education Center, Jay, FL), and Calvin Pierce and Marissa Nufer (USDA Agricultural Research Service) Southern Insect Management Research Unit, Stoneville, MS) for technical assistance with molecular cloning and DNA sequencing.
Conflict of interest
Author XLFR was employed by the company Syngenta Seeds. Author JWJ was employed by the company Syngenta Crop Protection Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hardwick DF. The corn earworm complex. Mem Entomol Soc Can (1965) 97:5–247. doi: 10.4039/entm9740fv
2. Pogue MG. A new synonym of Helicoverpa zea (Boddie) and differentiation of adult males of h. zea and H. armigera (Hubner) (Lepidoptera:Noctuidae:Heliothinae). Ann Entomol Soc Am (2004) 97:1222–6. doi: 10.1603/0013-8746(2004)097[1222:ANSOHZ]2.0.CO;2
3. Behere GT, Tay WT, Russell DA, Batterham P. Molecular markers to discriminate among four pest species of Helicoverpa (Lepidoptera: Noctuidae). Bull Entomol Res (2008) 98:599–603. doi: 10.1017/s0007485308005956
4. Tay WT, Soria KF, Walsh T, Thomazoni D, Silvie P, Behere GT, et al. A brave new world for an old-world pest: Helicoverpa armigera (Lepidoptera: Noctuidae) in Brazil. PloS One (2013) 8:11. doi: 10.1371/journal.pone.0080134
5. Perera OP, Allen KC, Jain D, Purcell M, Little NS, Luttrell RG. Rapid identification of Helicoverpa armigera and Helicoverpa zea (Lepidoptera: Noctuidae) using ribosomal RNA internal transcribed spacer 1. J Insect Sci (2015) 15:155. doi: 10.1093/jisesa/iev137
6. Behere GT, Tay WT, Russell DA, Heckel DG, Appleton BR, Kranthi KR, et al. Mitochondrial DNA analysis of field populations of Helicoverpa armigera (Lepidoptera: Noctuidae) and of its relationship to h. zea. BMC Evol Biol (2007) 7:117. doi: 10.1186/1471-2148-7-117
7. Reay-Jones FPF. Pest status and management of corn earworm (Lepidoptera: Noctuidae) in field corn in the united states. J Integr.Pest Manage (2019) 10:1–9. doi: 10.1093/jipm/pmz017
8. CABI Invasive Species Compendium. Helicoverpa armigera (Cotton bollworm) . Available at: https://www.cabi.org/isc/datasheet/26776 (Accessed February 8, 2022).
9. Kitching IJ, Rawlins JE. The noctuoidea. In: Kristensen NP, editor. Lepidoptera: moths and butterflies. New York: Walter de Gruyter (1998). p. 355–401.
10. Tembrock LR, Timm AE, Zink FA, Gilligan TM. Phylogeography of the recent expansion of Helicoverpa armigera (Lepidoptera: Noctuidae) in south America and the Caribbean basin. Ann Entomol Soc Am (2019) 112:388–401. doi: 10.1093/aesa/saz019
11. Czepak C, Cordeiro Albernaz K, Vivan LM, Oliveira Guimaraes H, Carvalhais T. First reported occurrence of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Brazil. Pesqui. Agropecu. Trop (2013) 43:110–3. doi: 10.1590/S1983-40632013000100015
12. Specht A, Sosa-Gomez DR, Paula-Moraes SV, Yano SAC. Identificação morfológica e molecular de Helicoverpa armigera (Lepidoptera: Noctuidae) e ampliação de seu registro de ocorrência no brasil. Pesquisa Agropecuaria Bras (2013) 48:689–92. doi: 10.1590/S0100-204X2013000600015
13. Sosa-Gomez DR, Specht A, Paula-Moraes SV, Lopes-Lima A, Yano SAC, Micheli A, et al. Timeline and geographical distribution of Helicoverpa armigera (Hubner) (Lepidoptera, noctuidae: Heliothinae) in Brazil. Rev Bras Entomol (2016) 60:101–4. doi: 10.1016/j.rbe.2015.09.008
14. ABC en el este. Senave en alerta tras ingreso de peligrosa plaga agrícola (2013). Available at: http://www.abc.com.py/edicion-impresa/economia/senave-en-alerta-trasingresode-peligrosa-plaga-agricola-629240.html (Accessed August 5, 2019).
15. Murua M, Cazado GLE, Casmuz A, Herrero MI, Villagrán ME, Vera A, et al. Species from the heliothinae complex (Lepidoptera: Noctuidae) in tucumán, Argentina, an update of geographical distribution of helicoverpa armigera. J Insect Sci (2016) 16:1–7. doi: 10.1093/jisesa/iew052
16. Arnemann JA, James WJ, Walsh TK, Guedes JVC, Smagghe G, Castiglioni E, et al. Mitochondrial DNA COI characterization of Helicoverpa armigera (Lepidoptera: Noctuidae) from Paraguay and Uruguay. Genet Mol Res (2016) 15:1–8. doi: 10.4238/gmr.15028292
17. Gilligan TM, Goldstein PZ, Timm AE, Farris R, Ledezma L, Cunningham AP. Identification of heliothine (Lepidoptera: Noctuidae) larvae intercepted at U.S. ports of entry from the new world. J Econ Entomol (2019) 1:13. doi: 10.1093/jee/toy402
18. Kriticos DJ, Ota N, Hutchison WD, Beddow J, Walsh T, Tay WT, et al. The potential distribution of invading Helicoverpa armigera in north America: Is it just a matter of time? PloS One (2015) 10:3. doi: 10.1371/journal.pone.0119618
19. North American Plant Protection Organization. Detection of old-world bollworm (Helicoverpa armigera) in Puerto Rico (2014). Available at: http://www.pestalert.org/oprDetail.cfm?oprID=600 (Accessed June 2019).
20. Smith E. Detection of old-world bollworm (Helicoverpa armigera) in Puerto Rico (2014). North American Plant Protection Organization, Phytosanitary Alert System Bulletin. Available at: http://www.pestalert.org/oprDetail.cfm?oprID=600 (Accessed March 8, 2022).
21. Hayden J, Brambila J. Pest alert: Helicoverpa armigera (Lepidoptera: Noctuidae), the old-world bollworm. Florida department of agriculture and consumer services (2015). Available at: http://www.freshfromflorida.com/Divisions-Offices/Plant-Industry/Plant-Industry-Publications/Pest-Alerts/Pest-Alert-The-Old-World-Bollworm (Accessed March 8, 2022).
22. Trujillo DX. Population monitoring and morphometrics of helicoverpa armigera (Hübner), helicoverpa zea (Boddie), and their hybrids (Lepidoptera: Noctuidae) in Puerto rico. dissertation. Mayaguez campus: University of Puerto Rico (2018).
23. Da Silva FR, Trujillo D, Bernardi O, Verle Rodrigues JC, Bailey WD, Gilligan TM, et al. Comparative toxicity of Helicoverpa armigera and Helicoverpa zea (Lepidoptera: Noctuidae) to selected insecticides. Insects (2020) 11:431. doi: 10.3390/insects11070431
24. USDA-NASS. Puerto Rico Agriculture. results from the 2018 census of agriculture . Available at: https://www.nass.usda.gov/Publications/Highlights/2020/census_puertorico.pdf (Accessed February 28, 2022).
25. Harstack AW, Witz JA, Buck DR. Moth traps for the tobacco budworm. J Econ Entomol (1979) 72:519–255. doi: 10.1093/jee/72.4.519
26. Jackson MD, Brown GC, Nordin GL, Johnson DW. Autodissemination of a baculovirus for tobacco budworm (Lepidoptera: Noctuidae) management on tobacco: A two-year, two-state field test. J Econ Entomol (1992) 85:710–9. doi: 10.1093/jee/85.3.710
27. Dow AgroSciences. Spinetoram technical bulletin (2006). Available at: http://www.dowagro.com/PublishedLiterature/dh_0072/0901b8038007298a.pdf (Accessed February 8, 2022).
28. Insecticide Resistance Action Committee (IRAC). Cotton bollworm, helicoverpa armigera . Available at: https://www.irac-online.org/pests/helicoverpaarmigera (Accessed 9 March 2021).
29. Zalucki MP, Daglish G, Firempong S, Twine PH. The biology and ecology of Heliothis armigera (Hubner) and h. punctigera wallengren (Lepidoptera: Noctuidae) in Australia: What do we know? Aust J Zool (1986) 34:779–814. doi: 10.1071/ZO9860779
30. Luttrell RG, Jackson RE. Helicoverpa zea and bt cotton in the united states. GM Crops Food (2012) 3:213–27. doi: 10.4161/gmcr.20742
31. Capinera JL. Featured creatures, in: Helicoverpa zea (Boddie) (Insecta: Lepidoptera: Noctuidae) (2000). Available at: https://entnemdept.ufl.edu/creatures/veg/corn_earworm.htm (Accessed March 11, 2022).
32. Stadelbacher EA. Role of early-season wild and naturalized host plants in the buildup of the F1 generation of Heliothis zea and h. virescens Delta Mississippi. Environ Entomol (1981) 10:766–70. doi: 10.1093/ee/10.5.766
33. Neuzig HH. Wild host plants of the corn earworm and the tobacco budworm in eastern north Carolina. J Econ Entomol (1963) 56:135–9. doi: 10.1093/jee/56.2.135
34. Zhudong L, Dianmo L, Peiyu G, Kunjun W. Life tables studies of the cotton bollworm, Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae), on different host plants. Environ Entomol (2004) 33:1570–6. doi: 10.1603/0046-225X-33.6.1570
35. Cunningham JP, Zalucki MP. Understanding heliohinae (Lepidoptera: Heliothinae) pests: What is a host plant? J Econ Entomol (2014) 3:881–96. doi: 10.1603/ec14036
36. Reigada C, Guimaraes KF, Parra JRP. Relative fitness of Helicoverpa armigera (Lepidoptera: Noctuidae) on seven host plants: A perspective for IPM in Brazil. J Insect Sci (2016) 16:3. doi: 10.1093/jisesa/iev158
37. Pomari-Fernandes A, de Freitas Bueno A, Sosa-Gomez DR. Helicoverpa armigera: current status and future perspective in Brazil. CAST (2015) 21:1–7. doi: 10.18539/cast.v21i1.4234
38. Wang HL, Zhao CH, Wang CZ. Comparative study of sex pheromone composition and biosynthesis in Helicoverpa armigera, h. assulta and their hybrid. Insect Biochem Mol Biol (2005) 35:575–83. doi: 10.1016/j.ibmb.2005.01.018
39. Guerrero S, Brambila J, Meagher RL. Efficacies of four pheromone-baited traps in capturing male Helicoverpa (Lepidoptera: Noctuidae) moths in northern Florida. Bio One complete. (2014) 97:1671–8. doi: 10.1653/024.097.0441
40. Shaw TJ, Paula-Moraes SV, Hahn PG, Specht A. Seasonal flight patterns of Chrysodeixis includens (Lepidoptera:Noctuidae) in the Florida panhandle and inventory of plusiine species cross-attracted to synthetic pheromone. J Econ Entomol (2021) 60:2315–25. doi: 10.1093/jee/toab179
41. Bentivenha JPF, Paula-Moraes SV, Baldin ELL, Specht A, da Silva IF. Battle in the new world: Helicoverpa armigera versus Helicoverpa zea (Lepidopera: Noctuidae). PloS One (2016) 11:12. doi: 10.1371/journal.pone.0167182
42. Laster ML, Hardee DD. Intermating compatibility between north American Helicoverpa zea and Heliothis armigera (Lepidoptera: Noctuidae) from Russia. J Econ Entomol (1995) 88:77–80. doi: 10.1093/jee/88.1.77
43. Leite NA, Alves-Pereira A, Correa AS, Zucchi MI, Omoto C. Demographics and genetic variability of the new world bollworm (Helicoverpa zea) and the old-world bollworm (Helicoverpa armigera) in Brazil. PloS One (2014) 9:11. doi: 10.1371/journal.pone.0113286
44. Anderson CJ, Oakeshott JG, Tay WT, Gordon KH, Zwick A, Walshn TK. Hybridization and gene flow in the mega-pest lineage of moth, Helicoverpa. Proc Natl Acad Sci U. S. A. (2018) 115:5034–3039. doi: 10.1073/pnas.1718831115
45. Cordeiro EMG, Pantoja-Gomez LM, de Paiva JB, Nascimento ARB, Omoto C, Michel AP, et al. Hybridization and introgression between Helicoverpa armigera and h. zea: an adaptational bridge. BMC Evol Biol (2020) 20:61. doi: 10.1186/s12862-020-01621-8
46. Specht A, Sosa-Gomez DR, Medeiros Rios DA, Matos Claudino VC, Paula-Moraes SV, Malaquias JV, et al. Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) in Brazil: the big outbreak monitored by light traps. Neotrop. Entomol (2021) 50:53–67. doi: 10.1007/s13744-020-00836-0
47. Mallet J. Invasive insect hybridizes with local pests. Proc Natl Acad Sci USA (2018) 115:4819–21. doi: 10.1073/pnas.1804081115
48. Jallow MFA, Cunningham JP, Zalucki MP. Intra-specific variation for host plant use in Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae): implications for management. Crop Prot (2004) 23:955–64. doi: 10.1016/j.cropro.2004.02.008
49. CABI Invasive Species Compendium. Helicoverpa zea (American cotton bollworm) . Available at: https://www.cabi.org/isc/datasheet/26757 (Accessed February 8, 2022).
50. Durigan MR. Resistance to pyrethroid and oxadiazine insecticides in helicoverpa armigera (Lepidoptera: Noctuidae) populations in brazil. Ph.D. dissertation. ESALQ, Brazil: University of Sao Paulo (2018).
51. Valencia-Montoya WA, Elfekih S, North HL, Meier JI, Warren IA, Tay WT, et al. Adaptive introgression across semipermeable species boundaries between local Helicoverpa zea and invasive Helicoverpa armigera moths. Mol Biol Evol (2020) 37:2568–83. doi: 10.1093/molbev/msaa108
52. Rios DAM, Specht A, Roque-Specht VR, Sosa-Gomez DR, Fochezato J, Malaquias JV, et al. Helicoverpa armigera and Helicoverpa zea hybridization: constraints, heterosis, and implications for the pest management. Pest Manage Sci (2022) 78:955–64. doi: 10.1002/ps.6705
53. Shapiro AM, Porter AH. The lock-and-key hypothesis: evolutionary and biosystematic interpretation of insect genitalia. Annu Rev Entomol (1989) 34:231–45. doi: 10.1146/annurev.en.34.010189.001311
54. Mikkola K. The lock-and-key mechanisms of the internal genitalia of the noctuidae (Lepidoptera): how are they selected for? Eur J Entomol (2008) 105:13–25. doi: 10.14411/eje.2008.002
55. Gutierréz-Moreno R, Mota-Sanchez D, Blanco CA, Whalon ME, Teran-Santofimio H, Rodriguez-Maciel JC, et al. Field-evolved resistance of the fall armyworm (Lepidoptera:Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J Econ Entomol (2019) 112:792–802. doi: 10.1093/jee/toy372
56. Lahm GP, Selby TP, Freudenberger JH, Stevenson TM, Myers BJ, Myers BJ, et al. Insecticidal anthranilic diamides: a new class of potent ryanodine receptor activators. Biorg Med Chem Lett (2005) 15:4898–906. doi: 10.1016/j.bmcl.2005.08.034
57. Ishaaya I, Kontsedalov S, Horowitz AR. Emamectin, a novel insecticide for controlling field crop pests. Pest Manage Science. (2002) 58:1091–5. doi: 10.1002/ps.535
58. Tian K, Dong L, Yiyang Y, Mei L, Xinghui Q. CYP6B6 is involved in esfenvalerate detoxification in the polyphagous lepidopteran pest, helicoverpa armigera. Pestic Biochem Physiol (2017) 138:51–6. doi: 10.1016/j.pestbp.2017.02.006
59. Jacobson A, Foster R, Krupke C, Hutchison W, Pittendrigh B, Weinzierl R. Resistance to pyrethroid insecticides in Helicoverpa zea (Lepidoptera: Noctuidae) in Indiana and Illinois. J Econ Entomol (2009) 102:2289–95. doi: 10.1603/029.102.0634
60. Rabelo MM, Paula-Moraes SV, Pereira EJG, Siegfried BD. Contrasting susceptibility of lepidopteran pests to diamide and pyrethroid insecticides in a region of overwintering and migratory intersection. Pest. Manage Sci (2020) 76:4240–7. doi: 10.1002/ps.5984
61. Tossou E, Tepa-Yotto G, Kpindou Douro OK, Sandeu R, Datinon B, Zeukeng F, et al. Susceptibility profiles of Helicoverpa armigera (Hubner) (Lepcioptera: Noctuidae) to deltamethrin reveal a contrast between the northern and southern Benin. Int J Environ Res Public Health (2019) 16:1882. doi: 10.3390/ijerph16111882
Keywords: Sex pheromone trapping, hybrids of Helicoverpa sp., invasive lepidopteran pests, Puerto Rico, IPM, IRM
Citation: Flores-Rivera XL, Paula-Moraes SV, Johnson JW, Jack CJ and Perera OP (2022) Helicoverpa genus on the edge of the continental U.S.: Flight phenology, analysis of hybrid presence, and insecticide performance in high-input field crops in Puerto Rico. Front. Insect Sci. 2:1010310. doi: 10.3389/finsc.2022.1010310
Received: 02 August 2022; Accepted: 17 October 2022;
Published: 03 November 2022.
Edited by:
Hugo A. Benítez, Universidad Católica del Maule, ChileReviewed by:
Bernard Moussian, Université Côte d’Azur, FranceWilliam D Hutchison, University of Minnesota Twin Cities United States
Copyright © 2022 Flores-Rivera, Paula-Moraes, Johnson, Jack and Perera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvana V. Paula-Moraes, paula.moraes@ufl.edu
†ORCID: Silvana V. Paula-Moraes, orcid.org/0000-0001-6888-2300
Cameron J. Jack, orcid.org/0000-0002-7066-3753
Omaththage P. Perera, orcid.org/0000-0002-0643-0066
 Xiomara L. Flores-Rivera
Xiomara L. Flores-Rivera Silvana V. Paula-Moraes
Silvana V. Paula-Moraes James W. Johnson
James W. Johnson Cameron J. Jack
Cameron J. Jack Omaththage P. Perera5†
Omaththage P. Perera5†